Uncovering the Secrets of the Smallest Phages
In a new paper in Nature Chemical Biology, IGI Affiliate Researchers Vivek Mutalik and Adam Arkin report advances in understanding phage biology that bring us closer to using these small predators to fight antibiotic-resistant bacteria. Mutalik talked with IGI’s Hope Henderson about their new findings.
WHAT DOES YOUR LAB STUDY?
We mainly study synthetic biology and functional genomics of microbes. Primarily, we develop tools to study bacteria, look at them in the microbiome context, and get them to do things. Along the way, I’ve become fascinated by bacteriophages
What are bacteriophages?

Bacteriophages, or phages for short, are viruses that infect bacteria.
How did you get interested in studying phages?
Phages are just amazing. Biology and engineering of phages has always been my dream to work on, but I never had an opportunity. In the last decade we developed a set of new technologies to study how phages interact with bacteria, and that led me into the field.
One of the things that I’ve wondered about phages is that they’re so small and so sneaky, but have developed such sophisticated genetic circuits to take control of the host and make copies of themselves. An arms race happens: the phage infects, and bacteria find a way to defend themselves, and the phage finds a new approach to counter attack. It’s kind of like hide and seek, you know? So I really like the discovery aspect about how these phage and host defense mechanisms have shaped this dynamic relationship
I’m also driven by the challenge of finding a solution to ever-rising antibiotic resistance across different places — from food manufacturing to hospitals. With no new antibiotics on the horizon, we urgently need to develop alternative therapies. Phages are one such very powerful alternative to antibiotics. Despite their importance, our efforts to characterize their interactions with target bacteria, the genes encoded on phages and phage resistance have been limited. By characterizing them systematically, we can come up with some smart ways to deploy them to mitigate dangerous bacterial infections. .
How could phages potentially be used to fight bacterial infections?
Phages infect and kill bacteria. So if we have a really nasty bacterial infection that’s not responding to any antibiotics, but we have phages that can kill that bacteria, then we have found an alternative option to eliminate that infection. Challenge is, as there is the arms race between bacteria and phages I mentioned earlier, bacteria evolve resistance to our phage all the time. So we need to come up with a new phage formulation to address this new bacterial mutant. So, we either need a huge collection of phages that target different receptors or a way to rapidly engineer phages so we have a bunch of phage variants when the need arises. We recently published an opinion piece on this and offer some new ideas on building a phage foundry to systematically characterize phages for not only bacterial infections in humans but also for use in diverse industries such as food, aquaculture, and meat industries, etc.
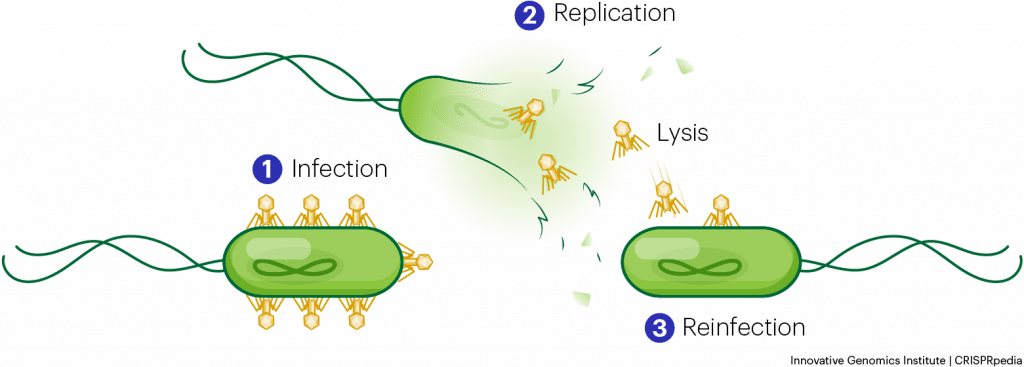
I think phages could be ideal for using in combination with other antimicrobial treatments. For example, there are some recent studies that indicate that if a bacteria is resistant to antibiotics, you can add phages, forcing the bacteria to evolve to survive. And that evolution to survive the phage attacks tends to make them vulnerable to antibiotics again. So you could imagine repeating that process in a patient with an infection! There are ongoing multiple clinical trials to assess the efficacy of phage therapy that we recently reviewed.
What kind of phages do you look at in your latest paper?
Our genetic material is in the form of double-stranded DNA. But viruses have more options. Some phages use double-stranded DNA, but others, like SARS-CoV-2 virus, use single-stranded RNA. Most phage research has been focused on double-stranded DNA phages.
Recently, with new genomic techniques, researchers have been able to collect all the RNA in a sample of water or soil and sequence it. And researchers have been finding tons of RNA phages everywhere from human gut to the ocean to soil. It has been known for a while that these kinds of phages exist, but we didn’t know how abundant they are, who they infect and how they impact their host or environment.
Many of these RNA phages are tiny. A human being has about 20,000 genes but these tiny phages have just four genes: one to make the outer shell, one to copy the RNA, one which helps in binding to the target bacteria or with transferring the RNA into the host cell, and a gene hidden somewhere along the genome that makes the host bacteria burst or ‘lyse.’ Just a handful of these ssRNA phages have been studied at molecular details.
How do RNA phages attack bacteria?
Well, studies based on a couple of model RNA phages, we know that they bind to hair-like structures called pili on the surface of bacteria and inject their genetic material into the target bacteria. However, we are limited by basic tools to answer these important and basic questions. We don’t even know how to isolate single-stranded RNA phages and we don’t even know which bacteria they infect or what they are doing! Essentially, we have more questions than answers to understand these smallest of phages.
So in this paper, we focused on one critical question: once the phage gets inside, how do the phages and their mysterious lysis proteins kill the host?
One of the collaborators on this paper, Ry Young, studies how phages induce lysis. His lab and

others found out that these small RNA phage genomes have a single gene that can cause lysis and that is sufficient to kill the host. They are also called “protein antibiotics” as they target the same proteins targeted by antibiotics.
But of the thousands of phages identified by genomics, we only know a few of these lysis genes, and only a couple of them have been studied at a molecular level. What proteins do they actually target once inside the target bacterial cell? What is the lysis mechanism? If we can answer those questions, we move towards being able to use them in therapies.
How do you start to understand phages better?
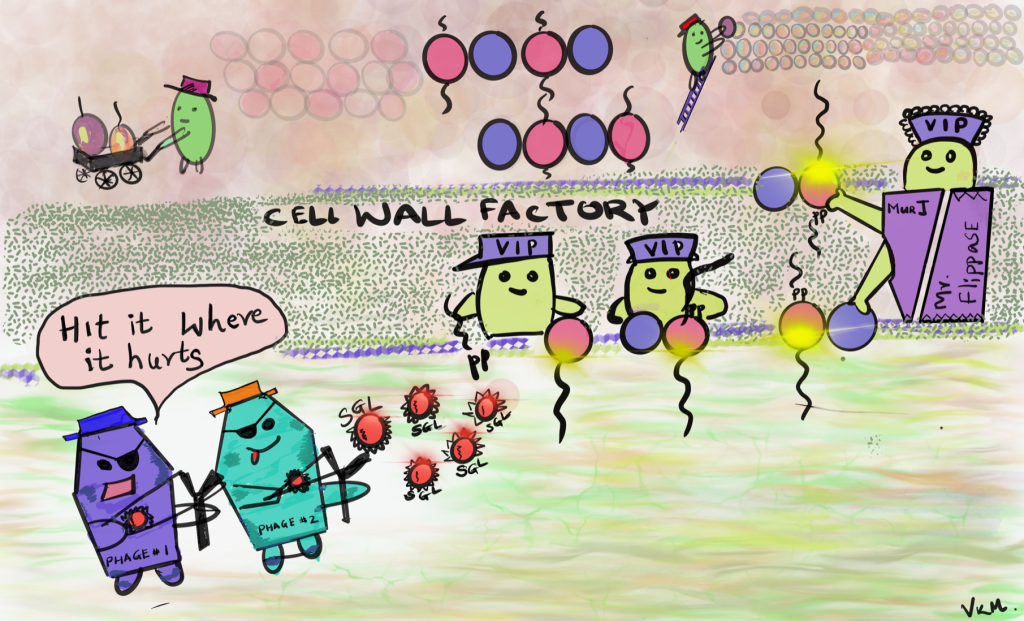
Ry and his team at Texas A&M are experts in phage lysis systems, and our team at IGI: Adam Arkin Lab at UC Berkeley, my lab and Adam Deutschbauer lab at LBNL brought our expertise in microbial genetics, phage expertise and high-throughput genomics technologies. In this paper, we worked together on a screen to identify which part of the bacteria the phages were targeting. For this work, we chose a collection of single gene lysis regions from diverse single-stranded RNA phages. We discovered potential targets for all of these lysis proteins. We followed up with one specific protein from Pseudomonas aeruginosa phage that targets a specific essential step in peptidoglycan or cell wall biosynthesis pathway important for bacterial survival. We know from earlier work that this same step is also targeted by another single gene lysis protein present on phage that infects E. coli. These both lysis proteins — their genetic sequences and protein structure are really different, but they target the same pathway for peptidoglycan or cell wall biosynthesis. So that’s really interesting! It’s an example of convergent evolution where single gene lysis proteins present on different phages targeting the same pathway to initiate lysis of the target bacteria. We think that we will see more such examples as we follow up with more such phage encoded single gene lysis proteins.
To go into it in more detail, this was a barcoded suppressor screen. We used genomic libraries where fragments of bacterial genomes have a genetic “barcode” added for quick and easy identification. These fragments were added to E.coli bacteria. So inside each bacterium, there are extra copies of whatever genes are on the fragment. Then, we expressed a single lysis protein in the cells — so, this is a phage protein that is toxic to the bacterial cells. And we see which bacterial cells survive.
The difference between ones that survive and ones that don’t is what genes are on the barcoded fragment. If extra copies of a gene help a bacterial cell survive longer, it’s a clue that that protein is involved in the lysis process. Once we identify which cells are survivors, we use the barcode to quickly identify what genomic fragment they had. And then we follow up some of these genetic screen results with additional experiments to validate the hits. You can read all the details in the paper!
Is there anything else you’d like to share?
We are really excited about the potential of this approach to study more phage genes. We can also extend this same screen to study small bacterial genes of unknown function known as “toxic genes” and uncover their molecular mechanisms.
This is an IGI-funded project, so I’m really thankful for IGI’s funding support. Ben Adler, currently transitioned to a postdoc position at Doudna lab, led this project during his Ph.D. thesis.
I am really thankful to our friends at Texas A&M for their collaboration, as well as DOE ENIGMA-SFA for funding the initial part of the project.
I have managed to get funding from the Berkeley Lab’s Laboratory Directed Research and Development (LDRD) program to continue working on ssRNA phages and all of their mysteries. For this I am partnering with Simon Roux of DOE Joint Genome Institute to look at all aspects of biology and engineering of non-model non-dsDNA phages.
 By
Hope Henderson
By
Hope Henderson

