IGI Researchers Develop Rapid, Point-of-Care COVID-19 Diagnostic Test
A new, sensitive saliva-based test would give results within an hour
Easy, rapid testing for COVID-19 is critical to controlling the spread of the virus, especially with more transmissible variants. IGI researchers have developed a new, sensitive spit-test method for detecting SARS-CoV-2, yielding results in an hour or less.
Today’s gold standard COVID-19 diagnostic test relies on PCR, a method for making copies of a piece of DNA or RNA. This test is highly sensitive, able to detect just one copy of viral RNA per microliter, but requires specialized equipment, refrigerated chemical components, a lab facility, and takes several hours to run. Typically, patients get results 24-48 hours after testing. Rapid antigen tests, or RATs, have become increasingly popular for their turnaround time of just 15-30 minutes, but are much less sensitive than PCR-based tests and can lead to false negatives.
A New Way to Test for COVID-19
A team led by IGI Founder Jennifer Doudna and IGI Investigators Patrick Hsu and David Savage has published a paper in Nature Biomedical Engineering showing a new prototype that uses a CRISPR enzyme to detect small amounts of SARS-CoV-2 RNA in an hour or less.
“The vision that Jennifer, Patrick, and Dave shared with us in the very beginning is that a workplace could have boxes at the front door. When you go in, you spit in a cartridge, put the cartridge in a box, and 30 minutes later you get a result on your phone,” says co-first author Sita Chandrasekaran, a graduate student researcher in the Hsu lab.

While the test, termed DISCovER, is not as sensitive as a conventional PCR-based COVID-19 diagnostic test, it is much more sensitive than RATs and could be used in similar settings — and the sample is taken by spitting in a tube, rather than with more invasive nasal swabbing.
“First, we wanted to build something that gives you a result quickly,” says co-first author Aditya Jangid, a Research Associate in the Hsu lab. “Second, we wanted to develop a test where you didn’t have to come into the clinic to give a sample and then wait for your results — the goal was to make a stand-alone system that could do everything.”
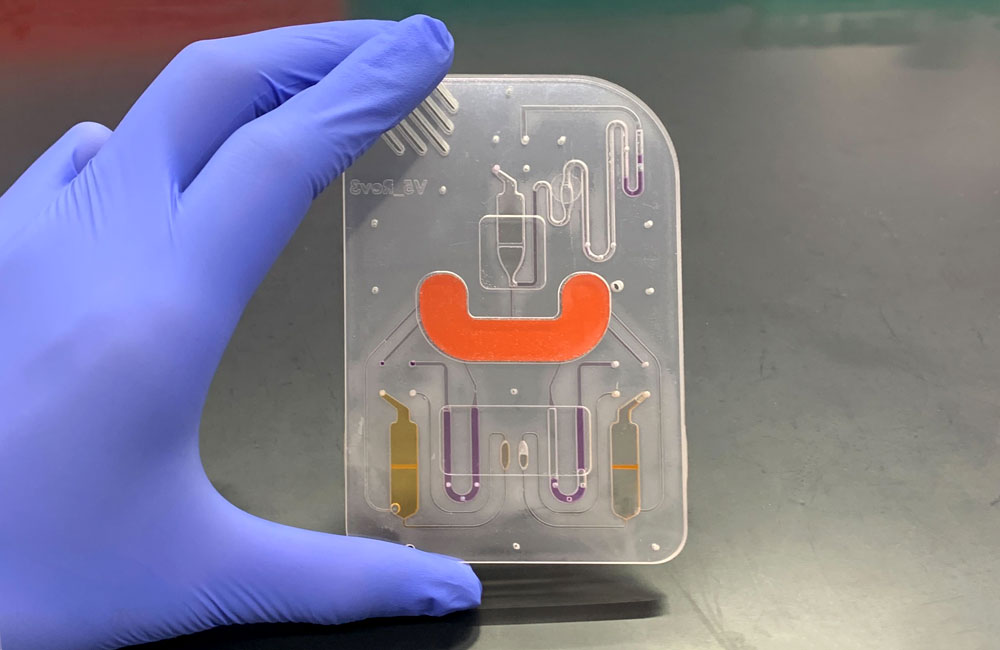
The DISCovER test, described today in Nature Biomedical Engineering, uses inexpensive, shelf-stable chemical solutions. The user provides a saliva sample to a microfluidic cartridge, which is then inserted into a compact device automated to process the sample and give a read-out. In the device, a process called LAMP, rather than conventional PCR, is used to make copies of any viral RNA present. LAMP can be performed at a constant temperature in a relatively short time, removing the need for expensive thermal cycling devices used in PCR. When viral RNA is present, a CRISPR-associated enzyme, Cas13, activates a fluorescent signal that serves as the test read-out.
The test is designed to detect any and all of the SARS-CoV-2 variants that have been seen so far, but the research has implications beyond COVID-19.
Beyond COVID-19

“One exciting aspect of the DISCoVER prototype is that it’s really a modular system,” says Patrick Hsu, an Assistant Professor at UC Berkeley and a cofounder of the Arc Institute in Palo Alto. “We could quickly and easily adapt this to test for any infection that can be detected in saliva. This type of point-of-care prototype could be developed to test for common infections like strep throat, mononucleosis, or herpesvirus infections, as well as for HIV, hepatitis viruses, measles, and more. Our goal is for individuals to be able to rapidly get results that they can discuss on the spot with their healthcare providers.”
This test is part of a growing world of CRISPR-based diagnostics that take advantage of the ability of CRISPR enzymes to locate specific genetic sequences, but instead of cutting DNA for genome editing the diagnostics trigger the release of a marker that indicates the presence of a specific pathogen. In addition to the DISCovER test, IGI researchers have also developed a test that requires no amplification, as well as a mobile phone-based device that can quickly detect the fluorescent markers from CRISPR-based tests working with researchers at Gladstone Institutes.
Media Contact:
Andy Murdock, andymurdock@berkeley.edu
 By
Hope Henderson
By
Hope Henderson