Megaphages Harbor Mini-Cas Proteins Ideal for Genome Editing
Megaphages harbor mini-Cas proteins ideal for genome editing
Berkeley News | Robert Sanders | July 16, 2020
UC Berkeley video by Roxanne Makasdjian
The DNA-cutting proteins central to CRISPR-Cas9 and related genome-editing tools originally came from bacteria, but a newfound variety of Cas proteins apparently evolved in viruses that infect bacteria.
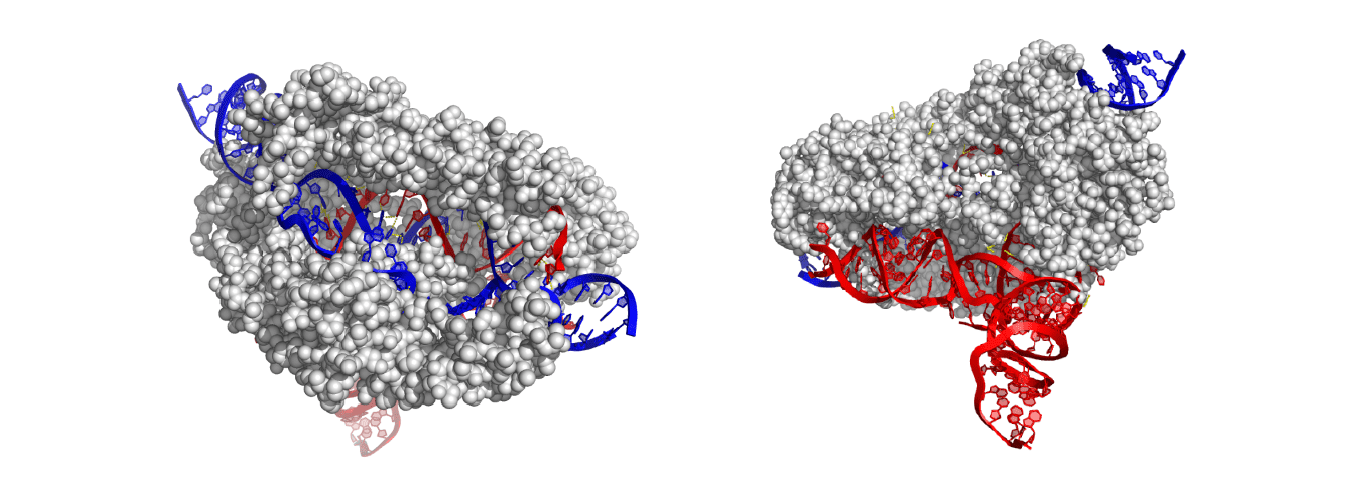
The new Cas proteins were found in the largest known bacteria-infecting viruses, called bacteriophages, and are the most compact working Cas variants yet discovered — half the size of today’s workhorse, Cas9.
Smaller and more compact Cas proteins are easier to ferry into cells to do genome editing, since they can be packed into small delivery vehicles, including one of the most popular: a deactivated virus called adeno-associated virus (AAV). Hypercompact Cas proteins also leave space inside AAV for additional cargo.
As one of the smallest Cas proteins known to date, the newly discovered CasΦ (Cas-phi) has advantages over current genome-editing tools when they must be delivered into cells to manipulate crop genes or cure human disease.
“Adenoviruses are the perfect Trojan horse for delivering gene editors: You can easily program the viruses to reach almost any part in the body,” said Patrick Pausch, a postdoctoral fellow at the University of California, Berkeley, and in UC Berkeley’s Innovative Genomics Institute (IGI), a joint UC Berkeley/UCSF research group devoted to discovering and studying novel tools for genome editing in agriculture and human diseases. “But you can only pack a really small Cas9 into such a virus to deliver it. If you would have other CRISPR-Cas systems that are really compact, compared to Cas9, that gives you enough space for additional elements: different proteins fused to the Cas protein, DNA repair templates or other factors that regulate the Cas protein and control the gene-editing outcome.”
Apparently these “megaphages” use the CasΦ protein — the Greek letter Φ, or phi, is used as shorthand for bacteriophages — to trick bacteria into fighting off rival viruses, instead of itself.
“The thing that actually made me interested in studying this protein specifically is that all the known CRISPR-Cas systems were originally discovered in bacteria and Archaea to fend off viruses, but this was the only time where a completely new type of CRISPR-Cas system was first found, and so far only found, in viral genomes,” said Basem Al-Shayeb, a doctoral student in the IGI. “That made us think about what could be different about this protein, and with that came a lot of interesting properties that we then found in the lab.”
Among these properties: CasΦ evolved to be streamlined, combining several functions in one protein so that it can dispense with half the protein segments of Cas9. It is as selective in targeting specific regions of DNA as the original Cas9 enzyme from bacteria, and just as efficient, and it works in bacteria, animal, and plant cells, making it a promising, broadly-applicable genome editor.
“This study shows that this virus-encoded CRISPR-Cas protein is actually very good at what it does, but it is a lot smaller, about half the size of Cas9,” said IGI founder Jennifer Doudna, a UC Berkeley Professor of Molecular and Cell Biology and of Chemistry and a Howard Hughes Medical Institute investigator. “That matters, because it might make it a lot easier to deliver it into cells than what we are finding with Cas9. When we think about how CRISPR will be applied in the future, that is really one of the most important bottlenecks to the field right now: delivery. We think this very tiny virus-encoded CRISPR-Cas system may be one way to break through that barrier.”
Pausch and Al-Shayeb are first authors of a paper describing CasΦ that appeared today in the journal Science.
Biggiephages carry their own Cas proteins
The CasΦ protein was first discovered last year by Al-Shayeb in the laboratory of Jill Banfield, a UC Berkeley Professor of Earth and Planetary Science and Environmental Science, Policy, and Management. The megaphages containing CasΦ were part of a group they dubbed Biggiephage and were found in a variety of environments, from vernal pools and water-saturated forest floors to cow manure lagoons.
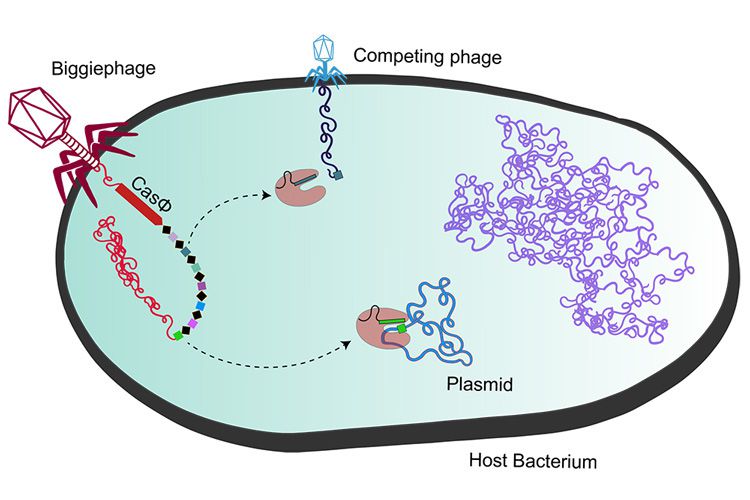
“We use metagenomic sequencing to discover the Bacteria, Archaea, and viruses in many different environments and then explore their gene inventories to understand how the organisms function independently and in combination within their communities,” Banfield said. “CRISPR-Cas systems on phage are a particularly interesting aspect of the interplay between viruses and their hosts.”
While metagenomics allowed the researchers to isolate the gene coding for CasΦ, its sequence told them only that it was a Cas protein in the Type V family, though evolutionarily distant from other Type V Cas proteins, such as Cas12a, CasX (Cas12e) and Cas14. They had no idea whether it was functional as an immune system against foreign DNA. The current study showed that, similar to Cas9, CasΦ targets and cleaves foreign genomes in bacterial cells, as well as double-stranded DNA in human embryonic kidney cells and cells of the plant Arabidopsis thaliana. It also can target a broader range of DNA sequences than can Cas9.
The ability of CasΦ to cut double-stranded DNA is a big plus. All other compact Cas proteins preferentially cut single-stranded DNA. So, while they may fit neatly into compact delivery systems like AAV, they are much less useful when editing DNA, which is double-stranded, inside cells.
As was the case after Cas9’s genome-editing prowess was first recognized in 2012, there is a lot of room for optimizing CasΦ for genome editing and discovering the best rules for designing guide RNAs to target specific genes, Pausch said.
Other co-authors of the paper are Ezra Bisom-Rapp, Connor Tsuchida, Brady Cress, and Gavin Knott of UC Berkeley, and Zheng Li and Steven E. Jacobsen of UCLA. The researchers were funded, in part, by the Paul G. Allen Frontiers Group, National Institutes of Health Somatic Cell Genome Editing consortium (U01AI142817-02), and National Science Foundation (DGE 1752814).
RELATED INFORMATION
- CRISPR-CasΦ from huge phages is a hypercompact genome editor (Science)
- Huge bacteria-eating viruses narrow gap between life and non-life (Feb. 12, 2020)
- Compact CRISPR systems found in some of world’s smallest microbes (Dec. 22, 2016)
Media Contact
Andy Murdock: andymurdock@berkeley.edu