*See our 2023 clinical trials review here.
2020 was a big year for CRISPR — the discoveries of new Cas proteins, use of CRISPR technology to study and develop diagnostic tests of COVID-19, a Nobel Prize, and more. The past year has also brought results from clinical trials using CRISPR technology, which we first reported on in 2019, and the start of new clinical trials.
In this article, we will go over the basics of clinical trials and then map out the current CRISPR-based trials from disease background to what we really hope to learn from these trials.
CLINICAL TRIAL BASICS
In the United States, the Food and Drug Administration (FDA) evaluates new disease treatments for safety and efficacy through clinical trials on patient volunteers. Early trials look at safety and side effects. Later trials test efficacy and compare new therapies with standard treatments.
The current trials using CRISPR-based treatments are still in early stages. That means that even if the treatments are safe and effective, they’re likely still a few years away from FDA approval and being broadly available to patients.
The advent of CRISPR technology opens up new possibilities in precision medicine. Current trials are underway in five treatment areas: blood disorders, cancers, eye disease, chronic infections, and protein-folding disorders. All current CRISPR clinical trials are intended to edit specific cells or tissues without affecting sperm or eggs, meaning no DNA changes can be passed onto future generations.
BLOOD DISORDERS
DISEASE BACKGROUNDS
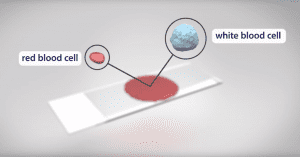
Red blood cells use hemoglobin to carry oxygen from the lungs to all the tissues of the body. Mutations in a gene that encodes part of the hemoglobin molecule cause two different genetic disorders: sickle cell disease (SCD) and beta thalassemia.
In sickle cell disease (SCD), red blood cells are misshapen. Their crescent or “sickle” shape makes them block blood vessels, slowing or stopping blood flow. This causes sudden, severe pain. Complications include chronic pain, organ damage, strokes, and anemia. In beta thalassemia, patients do not make enough hemoglobin. This leads to anemia and fatigue. In more severe cases, patients have organ damage, especially to the liver, bones, and heart. Both diseases can be fatal.
There are some treatments available, but often, patients still suffer severe symptoms and complications from their diseases. Patients with more severe SCD and beta thalassemia need frequent blood transfusions. Bone marrow transplant can be curative; however, this can only be done when a healthy, matching donor can be found. This is not an option for most SCD or beta thalassemia patients.
TREATMENT STRATEGIES
The approach taken to treat blood disorders with CRISPR technology doesn’t directly fix the gene variants that cause disease, but uses a clever workaround: instead of directly fixing the disease-causing mutations, the goal is to increase levels of fetal hemoglobin. This is a form of hemoglobin that fetuses make in the womb, but children and adults don’t make. It is not entirely understood why humans switch from one form of hemoglobin to the other, but fetal hemoglobin can take the place of defective adult hemoglobin in red blood cells. This treatment can be used to treat both beta thalassemia and SCD.
The first step of treatment is to harvest a patient’s blood stem cells from their blood. Next, scientists edit the genomes of these cells. Then, chemotherapy eliminates the defective blood stem cells in the patient’s body, and billions of genome-edited stem cells are put back into their bloodstream. The gene-edited blood stem cells are delivered by IV. If this approach works as intended, these cells will settle in and create a new blood stem cell population in the bone marrow, which will make edited red blood cells that produce fetal hemoglobin.
This treatment approach is considered ex vivo genome editing, because the editing occurs outside of the patient’s body. Ex vivo editing guarantees that genome-editing tools only come in contact with the right target cells.
CURRENT CRISPR CLINICAL TRIALS

In the first use of an ex vivo CRISPR-based therapy to treat a genetic disease, researchers treated a patient with beta thalassemia in Germany in February 2019. 12 more patients have since been treated, and seven of them have been followed for at least three months. None of the patients needed blood transfusions in the months after treatment. The first patient with SCD was treated with the same therapy in Nashville, Tennessee in July 2019. This patient, Victoria Gray, has shown remarkable progress. Hear from Gray herself. Early results on other patients are promising, too:
- All patients treated for SCD or beta thalassemia are showing normal to near-normal hemoglobin levels, where at least 30% (SCD) or 40% (beta thalassemia) of hemoglobin is fetal hemoglobin.
- In bone marrow samples taken from Gray, an additional SCD patient, and five beta thalassemia patients, researchers found cells with the expected genetic edit that allows them to make fetal hemoglobin. This indicates that the edited cells have successfully taken up residence in the bone marrow.
- The only immediate side effects associated with the treatment resulted from the administration of chemotherapy.
- Read more:
- CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia — Frangoul et al., New England Journal of Medicine
- Safety and Efficacy of CTX001 in Patients with Transfusion-Dependent β-Thalassemia and Sickle Cell Disease — Abstract from Frangoul et al. at the American Society of Hematology
CRISPR Therapeutics and Vertex Pharmaceuticals are jointly running the fetal hemoglobin trials, and recruiting patients in the US, Canada, and Europe.
A forthcoming trial from IGI, UCSF, UCLA, and UC Berkeley is planning to test an alternate approach that would directly repair the mutation that causes SCD.
WHAT TO WATCH FOR
Right now, there is a burst of research into treating blood disorders. Pharmaceutical and biotech companies as well as academic research institutions are working on both pharmaceutical and genetic therapies. We don’t know yet which approaches will be the safest and most effective, but patients are sure to benefit from the increased research activity in these disease areas.
The initial results from Victoria Gray and other patient-volunteers are extremely encouraging. We look forward to more detailed results from other trial participants, who will hopefully show similar symptom reduction. Long-term follow-up of Gray and other patients is crucial: they will be tracked for years to come to see if the treatment remains effective, and to look for potential side effects, like negative health effects or cancer from unwanted or off-target edits, which won’t be apparent until further down the line.
“The main side effects so far have been from the chemotherapy necessary to wipe out the pre-existing bone marrow cells in order for the edited cells to engraft. The chemotherapy is a huge limiting factor for these therapies,” explains Megan Hochstrasser, Ph.D., a CRISPR expert who studied with Jennifer Doudna and now serves as the IGI Education Program Manager. “If you have to be in the hospital for weeks because you are getting your bone marrow ablated with chemotherapy, which cripples your immune system, it’s risky, expensive, and time-consuming. That’s a huge barrier to scaling this and making it available widely. It’s a big hurdle that could be overcome if someone finds a way to deliver the treatment directly, without bone marrow ablation.”
Scalability — getting the treatment to the many people who need it — will be a major challenge if the treatment move forward to FDA approval, both because of the technical challenges of creating the individualized product and administering the treatment protocol, and the cost. Research into in vivo approaches, which could eliminate the need for chemotherapy and decrease the associated risks and expenses, is in early stages, but will be a focus of those working to make more widely accessible CRISPR-based therapies for blood disorders in the coming years.
CANCERS
DISEASE BACKGROUND
Cancer refers to a group of diseases that are caused by uncontrolled cell growth. Right now, CRISPR-based therapies are mainly aimed at treating blood cancers like leukemia and lymphoma. A trial in China for a type of lung cancer was recently completed, as well.
TREATMENT STRATEGY
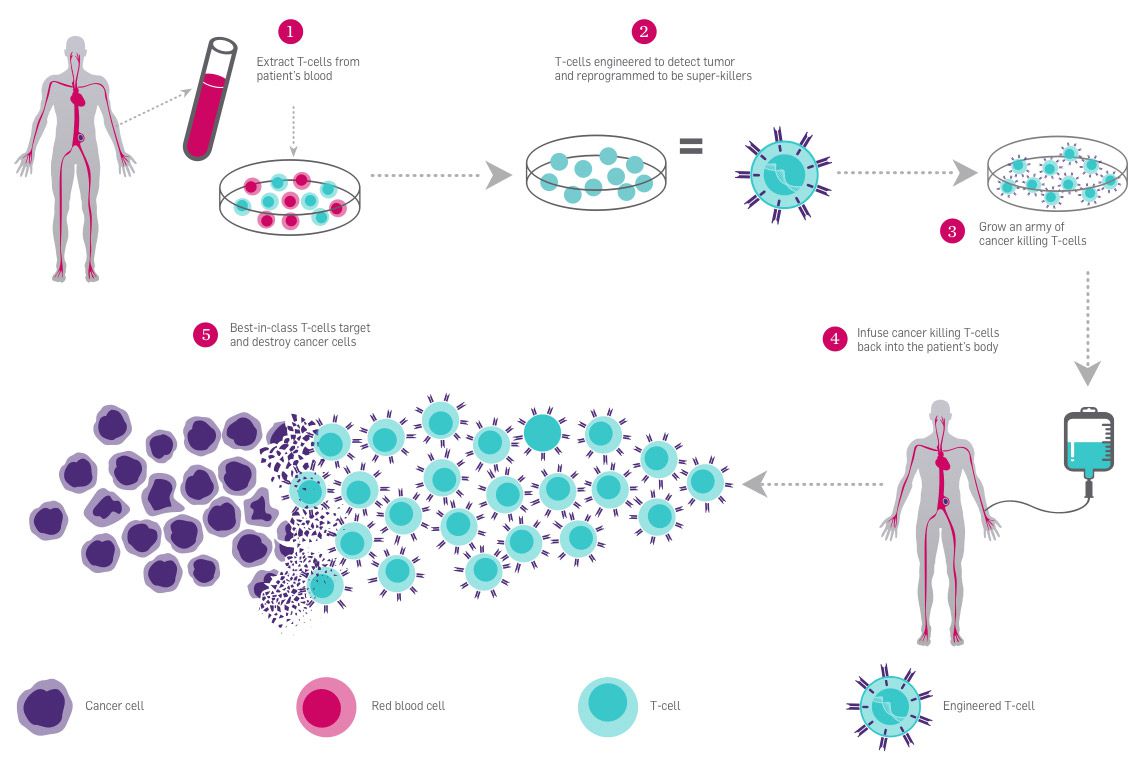
T cells, a type of white blood cell essential for immune system response, are covered in receptors that recognize other cells as safe or threatening. They patrol the body, killing foreign or dangerous cells, or recruiting other cells to assist. In CAR-T immunotherapy, researchers genetically engineer a patient’s T cells to have a receptor that recognizes the patient’s cancer cells, telling the T cells to attack. The immune system is highly regulated to avoid attacking healthy cells. Some T cell receptors work as “checkpoints” that determine whether an immune response occurs. When a T cell PD-1 receptor comes in contact with a molecule called PD-L1 on another cell, it communicates that it is a “safe” cell and the T cell leaves it alone.
Cancer cells are often cloaked in these molecular safety signals, tricking the patrolling T cells into ignoring them. Researchers are using CRISPR to edit the PD-1 gene in T cells to stop them from making functional PD-1 receptors so they can’t be fooled by cancer cells. This immunotherapy approach is known as checkpoint inhibition, and it is often used in conjunction with CAR-T engineering to give T cells the greatest possible chance of eliminating cancer.
For these treatments, researchers harvest T cells from a patient’s blood and engineer them in a lab. Then, they put them back into the patient’s bloodstream by IV. Because this treatment relies on ex vivo editing, it is easy to deliver the genome-editing tools to the target cells. CAR-T therapy was approved for use in treating blood cancers in 2017.
Learn more about CAR-T:
- CAR T-cell Therapy and Its Side Effects — The American Cancer Society
- CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers — National Cancer Institute
CURRENT CRISPR CLINICAL TRIALS
In 2016, a lung cancer patient became the first person in the world to be treated with a CRISPR therapy: this patient was injected with PD-1 edited T cells in a Chinese clinical trial. This and an American clinical trial using CRISPR-based immunotherapies for cancer have been completed. Several other clinical trials using CRISPR-based immunotherapies, mainly to treat blood cancers, are ongoing.
In the Chinese study, researchers at the West China Hospital, Sichuan University treated 12 patients with non-small-cell lung cancer with PD-1 edited T cells. This approach did not include CAR-T, as it is not currently an option for lung cancers. The main goals of this study were to test whether the treatment was safe, had tolerable side effects, and whether it elicited a dangerous immune response.
In April 2020, results from this trial were published in Nature Medicine. The reported findings include:
- The treatment was safe to administer and had acceptable side effects like fever, rash, and fatigue.
- The desired edit was found in a median of 6% of T cells/patient before infusion back into the patient.
- Off-target effects — unwanted changes at various places in the genome — were observed at a low frequency and were mostly in parts of the genome that don’t code for proteins. On-target effects — unwanted changes at the target site — were more common (median of 1.69%).
- Edited T cells were found in 11 out of 12 patients two months after the infusion, although at low levels. Patients with higher levels of edited cells had less disease progression.
- Read more:
- Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer — Yu et al., Nature Medicine
- First Trial of CRISPR-Edited T cells in Lung Cancer — Lacey & Fraietta, Trends in Molecular Medicine
The first CRISPR-based therapy trial in the US combined CAR-T and PD-1 immunotherapy approaches, using CRISPR to edit a total of three genes. This phase 1 study, run by the University of Pennsylvania in conjunction with the Parker Institute, began recruiting in 2018 and was completed in February 2020. Like the Chinese trial, the goals were to determine if the treatment was safe and had acceptable side-effects, not to cure patients. Two patient volunteers with advanced white blood cell cancer (myeloma) and one with metastatic bone cancer (sarcoma) were treated. The reported findings are:
- The treatment was safe to administer and had acceptable side effects.
- The T cells took up residency in the bone marrow and remained at stable levels for the nine months of the study.
- Tumor biopsies showed that T cells were able to find and infiltrate tumors.
- Off-target effects were rarely observed. But, unwanted changes at the target site were observed frequently, with 70% of cells showing at least one mutation at or near the target site during manufacturing. After infusion and over time in patients, the percentage of cells with mutations decreased.
- Read more:
- CRISPR-engineered T cells in patients with refractory cancer — Stadtmauer et al., Science
Together, these studies indicate that CRISPR-engineered CAR-T therapy may be a promising line of treatment: it is safe, the side effects are tolerable, and the treatment does not induce a strong immune reaction.
Neither treatment provided a cure, but the aim of these studies was only to see if the treatment was safe and should be developed further for treatment, not to cure disease. As first attempts, both trials met their goals of showing safety and tolerability.
“A really interesting thing is that the American study did show a percentage of the large genomic rearrangements that people fear,” says Hochstrasser. “But the percentage of cells with these changes actually decreased over time. It seemed like the cells that had those types of mutations were dying or getting out-competed by the other cells. So, it seemed like the cells that you wouldn’t want in the body were not actually sticking around in the body, which was a surprise to me, and very encouraging.”
WHAT TO WATCH FOR
The FDA has already approved CAR-T therapies and PD-1 pathway inhibitors that don’t use genome editing. This is a reason for optimism: the proof-of-principle work for these therapies has already been done successfully.
According to Hochstrasser, “The efficiency of editing — meaning, the percentage of cells that actually got edits — wasn’t great in either trial. But these trials were approved years ago, and done using technology from 2016. There have been many advancements in the technology since then. So, it’s an important proof of concept around the immediate safety and tolerability of the treatment, and we’ll have to see if they can increase editing efficiency.”
Using now-available, more potent techniques, will editing efficiency be higher? And with higher editing efficiency, will genetic checkpoint inhibition work as well or better than checkpoint-blocking drugs? Will PD-1 editing be as or more effective than antibody treatments that disable PD-1? Future research will have to answer these questions.
EYE DISEASE
DISEASE BACKGROUND
Leber Congenital Amaurosis (LCA) is the most common cause of inherited childhood blindness, and LCA10 is the most common form of LCA. This disease, caused by a single nucleotide mutation in a photoreceptor gene, causes severe vision loss or blindness within the first few months of life.
Photoreceptor cells in the eye convert light into nerve signals that travel to the brain. In LCA10, the mutation in a photoreceptor gene means cells make a shortened, defective version of a crucial protein. This defective protein makes the photoreceptor cells dysfunctional. Patients with LCA10 can receive light to the eye, but the dysfunctional photoreceptor cells can’t send messages to the brain. Without communication between the eyes and the brain, patients have vision loss or blindness.
TREATMENT STRATEGY
The CRISPR treatment for LCA10 makes a change to the patient’s faulty photoreceptor gene so that it once again makes a full-size, functional protein instead of the short, broken version. If enough cells are edited to make the healthy protein, patients are expected to regain vision.
Patient volunteers receive a single dose of the CRISPR therapy by injection directly into the eye. The injection contains a nonpathogenic virus (AAV) carrying the Cas9 protein and its guide RNA. Viruses are often used in gene therapy and genome editing because they have a natural ability to get into cells. For the LCA10 treatment, the viral vector is engineered so the therapy is active only in photoreceptor cells.
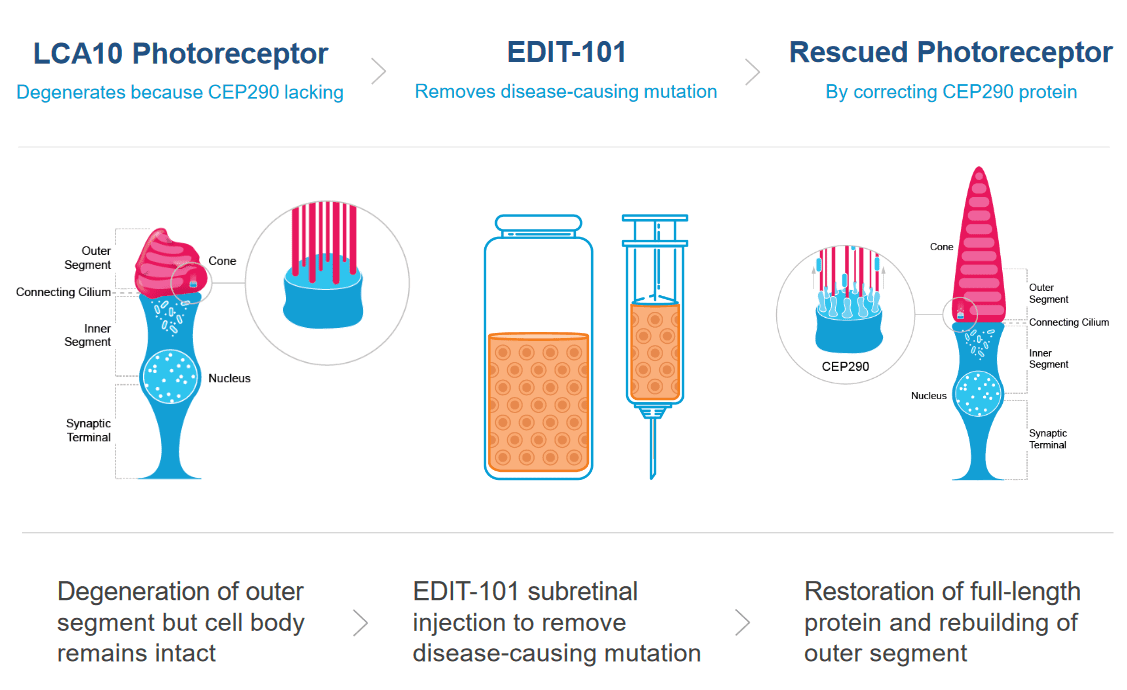
This approach is distinct from those being tested for cancer and blood disorders because it is an in vivo treatment, meaning the genome editing occurs inside the patient’s body. Compared to ex vivo editing, in vivo editing has more challenges and a different set of risks. One of the biggest risks is that viral delivery tools or genome editing components will provoke dangerous immune reactions in a patient. Another big challenge is making sure that CRISPR enzymes don’t stick around for too long, since that would give them a greater chance of cutting in unwanted places in the DNA.
You may feel squeamish thinking about needles near eyeballs, but the eye is actually an ideal organ for in vivo editing. It is small, so it only requires a single-dose, small-volume treatment. The eye has less immune reactivity than most tissues, making a dangerous immune reaction less likely to occur. Because the eye is relatively contained, the CRISPR components aren’t likely to travel to other parts of the body, so there is a lower risk of unwanted genome editing or immune responses in other tissues. In experiments on a mouse model of LCA with the same mutation, researchers found that about 10% of cells showed the desired edit — this is thought to be the percent needed to get vision improvement. The treatment showed few side-effects in animal models, and studies in human retinal cells showed no off-target effects at over 100 sites with similar sequences. See preclinical data here.
CURRENT CRISPR CLINICAL TRIAL
This is the first in vivo CRISPR therapy trial, meaning, the first time CRISPR is being used to edit someone’s genes within their own body. The first patient-volunteer in this study, sponsored by Editas Medicine, was given a low-dose of the treatment in March 2020. Dosing of all patients in the first, low-dose cohort was completed by November 2020. A medium-dose cohort is expected to be treated soon. No results have been released yet.
WHAT TO WATCH FOR
Researchers will need to follow patients closely to see if cells other than the photoreceptor cells they’re targeting are affected, or if patients have immune reactions to the treatment. Other key research questions include: what percentage of cells will get effectively edited? Will it be enough for patients to regain vision?
If the treatment works, it will be the first trial leading to a direct fix for a genetic disease. Traditional gene therapies, like the ones used for severe combined immunodeficiency, commonly known as “bubble boy disease,” work by adding an extra gene — not by fixing the faulty gene. Likewise, the first CRISPR-based treatments for SCD and beta thalassemia don’t fix the original, disease-causing mutation. Success in this trial would be a major step forward in genetic medicine.
CHRONIC INFECTION
DISEASE BACKGROUND
Urinary tract infections (UTIs) are a common infection causing over eight million visits to health care providers every year. UTIs occur when bacteria that shouldn’t be there take up residence in the bladder, kidneys, the tubes that connect the bladders to the kidneys, or the tube through which urine exits the body. E. coli, a common fecal bacteria, is usually the culprit. UTIs are much more common in women for anatomical reasons.
UTIs cause a burning sensation during urination and the need to urinate frequently. Beyond discomfort, they can become dangerous if they affect the kidneys or if bacteria enter the bloodstream. Most are easily treated with a short course of antibiotics, but sometimes antibiotics are ineffective or the infections keep recurring. This is referred to as chronic UTI.
TREATMENT STRATEGY
This treatment is a cocktail of three bacteriophages combined with CRISPR-Cas3 to attack the genome of the three strains of E. coli responsible for about 95% of UTIs. The destruction of the genome kills the bacteria.
Bacteriophages, or phages for short, are viruses that attack bacteria. They usually work by injecting their genetic material into bacteria and using the bacteria as factory to make more bacteriophages. Eventually, the bacteria will burst, dying as it releases more copies of the phage. Phages are being developed for use against bacterial infections, and have gotten more attention recently as antibiotic resistance has become a major public health threat.
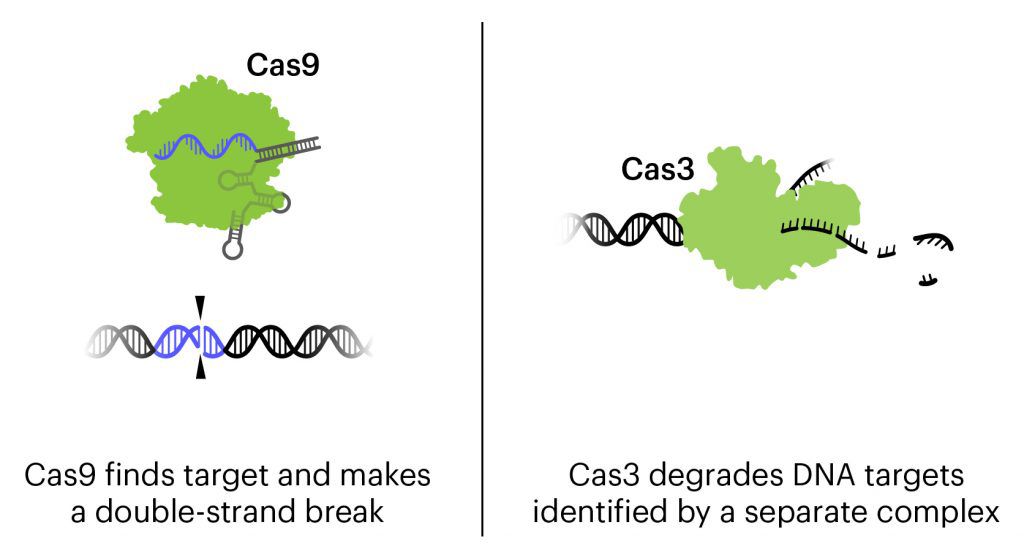
In this treatment, phages have been engineered to be an even more powerful tool against E. coli. In addition to the natural action of phages that kills bacteria, these bacteriophages contain CRISPR-Cas3 in their genome. While the more-famous Cas protein Cas9 makes a precise cut at a single location, Cas3 shreds DNA at the gene regions it is targeted to find. In this treatment, the CRISPR-Cas3 system is made to target the genomes of the targeted E. coli strains and damage them by shredding stretches of DNA. In experiments on isolated cells and in animal experiments, the addition of CRISPR-Cas3 makes phages much more effective at killing E. coli. Locus Biosciences delivered the treatment directly to the bladder by catheter in the phase 1 trial, and plans to develop an oral or IV treatment for later phases.
CURRENT CRISPR CLINICAL TRIAL
This is the first trial using a CRISPR-based therapy to treat infection. It is also the first trial to use the Cas3 protein, which targets longer stretches of DNA for destruction, rather than Cas9, which makes a precise cut at one location.
Phages have been considered a possible antibacterial therapy since they were first identified about 100 years ago. The discovery of antibiotics like penicillin soon after, as well as the difficulty of patenting phages, has limited the development and testing of phage therapies. In the time since, phages have occasionally been used by doctors for is known as “compassionate treatment.” Compassionate drug use or compassionate treatment is when an unapproved drug or therapy is used to treat a seriously ill patient as a last resort when no other treatments exist. At least 25 case reports of compassionate phage therapy have been published in the last 20 years. Some cases claim success at healing patients, but reports use different phages in different amounts for different conditions — clinical trials will be necessary to systematically evaluate the safety and efficacy of phage treatments.
As resistance to traditional antibiotics like penicillin becomes a major public health threat, there is growing interest in developing and testing phage therapies. Phages could in some ways even be preferable to antibiotics that are effective, because each phage usually only kills a specific bacteria. So, instead of being treated with an antibiotic that is destructive to healthy bacteria as well, phage therapy is ideally much more targeted and precise. In addition to innovations using CRISPR technology, this trial is significant because it is one of the first few well-controlled clinical trials for phage therapy, and the first to combine the CRISPR system with phage therapy.
Locus Biosciences began recruiting patient volunteers in the United States at the end of 2019, and completed their Phase 1b trial in February, 2021. Locus Biosciences reported that results of the trial supported the safety and tolerability of the new therapy, with no drug-related adverse effects. You can learn more in this interview with Joseph Nixon of Locus Biosciences.
WHAT TO WATCH FOR
The completed trial was Phase 1, which means it was primarily designed to test whether the treatment is safe and has tolerable side effects, not how effective the treatment is. However, the results from the trial indicate that the therapy can decrease the level of E. coli in the bladders of infected patients. Locus Biosciences plans to move forward with Phase 2 human efficacy trials, but has not yet announced a date.
CRISPR expert Megan Hochstrasser is interested in what the trial might reveal about the Cas3 protein: “Cas3 is like a lawn mower that plows through DNA and cuts it up. Cas9, on the other hand, makes a double-stranded break in the DNA. The bacteria should be killed with either kind of damage, but perhaps Cas3 could better prevent any straggling survivors that somehow managed to repair the Cas9 cut. But I’d be curious to know if there is a difference, because it’s part of a huge system that could be hard to deliver in other contexts.”
RARE PROTEIN-FOLDING DISEASE
DISEASE BACKGROUND
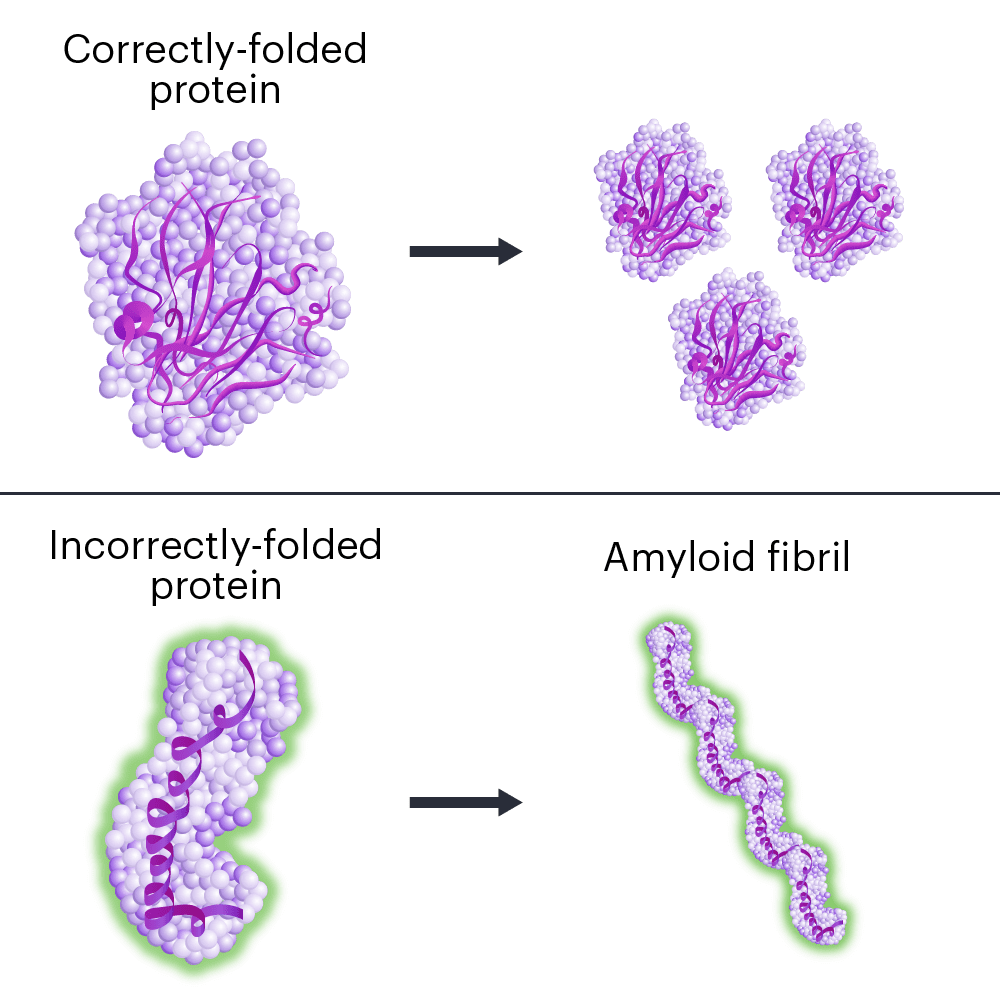
Hereditary transthyretin amyloidosis (hATTR) is a fatal disease caused by mutations in a single DNA letter in the gene TTR.
When the TTR gene is mutated, the protein it makes folds the wrong way. Incorrectly folded proteins stick together and form clumps called amyloid fibrils, in a process called amyloidosis. The protein clumps accumulate in organs and tissues, interfering with their normal functions.
ATTR first affects patients in early or middle adulthood. Symptoms vary, but ATTR usually has severe effects on the nervous system and/or heart. Nerve pain, loss of movement control, digestive problems, vision loss, dementia, and heart failure are common in ATTR. The effects on the nervous system and/or heart eventually kill patients.
ATTR usually occurs spontaneously, but for some patients, the mutated gene is passed down from their parents. This leads to hereditary ATTR, or hATTR. hATTR is a rare disease, affecting about 50,000 people worldwide.
Often, hereditary forms of neurological diseases are better understood than spontaneous cases because they are easier to study. ATTR has similarities to other neurological diseases involving protein misfolding and amyloidosis including Alzheimer’s and Parkinson’s diseases.
TREATMENT APPROACH
This treatment uses CRISPR-Cas9 tools to reduce the amount of faulty TTR protein the body makes. Less faulty TTR means less formation and accumulation of protein clumps (amyloidosis). The treatment is delivered in a single dose by IV.
The goal of hATTR treatment isn’t to fix a gene: it’s to break the gene so that patients stop making the faulty protein altogether. The CRISPR components cut the TTR gene, creating a double-stranded break in the DNA. As the cell tries to repair the DNA without a corrected template, the repair attempts mutate the gene even more. And when a gene is too badly damaged, a cell will stop making the protein it codes for.
Getting the CRISPR components into cells is a major hurdle for in vivo genome-editing therapies. Many treatments in development use viruses to deliver the genome-editing components. This will be the first clinical trial for a CRISPR-Cas9 therapy delivered in a lipid nanoparticle. The lipids, or fat molecules, surround the gene-editing components, and are able to get into the cell.
In animal models, lipid nanoparticles tend to accumulate in the liver. TTR is primarily made in the liver, so researchers are taking advantage of this tendency to naturally get the treatment to where it is needed. See preclinical data here.
CURRENT CRISPR CLINICAL TRIAL
This is the first trial using lipid nanoparticles to deliver the genome-editing treatment. It is also the first trial to deliver genome-editing components systemically, meaning to the whole body rather than to one specific type of cell or tissue.
The trial is sponsored by Intellia Therapeutics in conjunction with Regeneron Pharmaceuticals. The first patient was dosed in November 2020 in the United Kingdom. Right now, more patient-volunteers with neurological symptoms are being recruited in the UK, and they hope to expand to other countries. The researchers plan to enroll up to 38 participants with neurological symptoms for the first part of the trial, and then expand to enroll additional patients with heart symptoms.
WHAT TO WATCH FOR
This is the first experimental CRISPR therapy to be administered systemically to edit genes inside the human body. Other treatments edit specific cell types outside of the body, and then put them back in after editing (like treatments for blood disorders and cancer) or deliver the genome-editing therapies to self-contained organs (like treatment for genetic blindness and chronic UTI). These strategies help ensure that edits only occur in the cells or tissue of interest.
One long-standing concern about genome-editing therapies is off-target effects: incorrect edits made by the genome-editing components. This is particularly a concern with CRISPR treatments delivered by viruses, since the genome-editing components may persist in the cell for a long time, giving them more opportunity to make editing errors. Avoiding systemic delivery helps reduce the overall risk.
In this trial, delivery is systemic, which could lead to greater risk of off-targets because more tissues are exposed. However, exposure risks are reduced because 1) lipid nanoparticles tend to aggregate in the liver, which is the tissue of interest in this disease and 2) no viruses are used to deliver the genome-editing components. In animal models of the hATTR treatment delivered by lipid nanoparticles, the genome-editing components were cleared from the body in less than a week, dramatically reducing the chance of off-target effects. Systemic administration of editing reagents also poses the risk of triggering a potentially dangerous immune reaction. Doctors will monitor patient-volunteers closely for these reactions.
Efficiency — meaning, what percentage of cells are edited — is a big question. In nonhuman primates, only 35 or 40% of liver cells need to be edited to reduce TTR levels enough to have a therapeutic benefit. Researchers will need to follow patients closely to see what percentage of liver cells are effectively edited, and what percentage is necessary to see a benefit.
“It will be a commentary on lipid nanoparticles and how promising they are for editing the liver,” says IGI CRISPR expert Megan Hochstrasser. “It might be a situation where we don’t actually need a very high percentage of cells to get edited to start to see a positive benefit for the patient, which would be great.”
THE BIG PICTURE: WHAT WE HOPE TO LEARN
Taken together, these CRISPR clinical trials are helping scientists learn about the types of DNA changes CRISPR enzymes make in different cells, including unwanted off-target changes and problematic on-target changes; the way the immune system reacts to CRISPR-Cas tools; and how well different delivery methods work. These trials are not a major test of what CRISPR can do, but of how well it does what it does.
Over the last year, there has been encouraging news: Victoria Gray and others seem to be functionally cured of sickle cell disease or beta thalassemia, and the edited cells have taken up residence in the bone marrow, indicating the potential for a long-lasting cure. Trials for cancer therapies are at early stages, but the safety and tolerability of the treatments looks promising for moving forward with more current editing technology. New trials initiated this year widen the scope of CRISPR applications and delivery methods.
Editing efficiency varied between trials, with those started more recently having higher efficiency. Differences in technology — newer CRISPR tools are more efficient — and delivery methods account for these differences. Researchers are continuing to develop new CRISPR editors and expanding delivery options with the aim of increased efficiency.
These studies did show unwanted edits to cells, including on- and off-target effects. In the short-term, they don’t seem to cause trouble. But will unwanted DNA changes have meaningful effects on patients down the line or when given to larger cohorts? And how will potential side effects compare to the severity of the diseases being treated? Patient-volunteers will have to be followed over the coming years to get a more complete understanding of the safety profile of these treatments.
We are starting to see more in vivo editing with the addition of the trials for chronic UTI and hATTR, and preclinical research indicates that more will be coming in the years ahead. The hATTR treatment stands out as the first treatment to be administered systemically: other in vivo treatments are delivered directly to specific, contained organs, or cells are edited outside of the body and then returned to the patient. The systemic introduction of genome-editing components is a big advance, and we’ll be watching this one closely.
What the current CRISPR clinical trials have in common are easier delivery options. Getting the treatments where they need to be, and only where they need to be, is one of the biggest challenges of CRISPR-based therapies. The trials initiated so far rely on convenient solutions to delivery, but as delivery improves the menu of options will expand significantly.
MORE MEDICAL CRISPR FIRSTS TO LOOK OUT FOR
While the current CRISPR clinical trials are exciting, they rely on making edits to DNA that are easy for CRISPR-Cas enzymes — they are not really major tests of what CRISPR technology itself can do. In terms of understanding the further reaches of CRISPR’s potential, we’ll learn a lot from future milestones:
- A CRISPR treatment that involves inserting DNA to repair or replace a faulty sequence, in essence “pasting” in new material, is still a big challenge.
- A CRISPR therapy that repairs multiple genes at once. Many common conditions, like diabetes and heart disease, are “polygenic,” meaning multiple genes play a role in their development. While researchers have achieved impressive feats in isolated cells and animal models, we’re a long
way from making multiple changes to the genome in real patients.
- A trial where CRISPR tools are used to turn genes on and off without editing the DNA sequence. These strategies, known as CRISPR activation and inhibition, don’t require making breaks in a patient’s DNA, so they might be safer. But, it’s unclear how long their effects would last in humans.
- A treatment that uses base editing. Base editing uses CRISPR components to directly change single DNA letters without making breaks in the DNA. For diseases caused by single-letter changes to DNA, base editors may be a safer editing option than CRISPR.
MORE INFO ON CRISPR CLINICAL TRIALS
If you or a loved one are interested in participating in a clinical trial, you can learn more about how US clinical trials work and how to find them on our Patients & Families page. Discuss all important medical decisions with your doctor. Keep in mind that clinical trials are the first tests of new medical treatments, so they are inherently risky to patients and never guaranteed to be successful.
Special thanks to Bruce Conklin, Luke Gilbert, Megan Hochstrasser, and Vivek Mutalik for fact-checking sections of this article.
 By
Hope Henderson
By
Hope Henderson
Hope Henderson holds a B.A. in Biology from Brown University and a Ph.D. in Molecular & Cell Biology from the University of California, Berkeley. She joined the IGI in 2019 to work in science communication. In addition to serving as IGI’s main writer, she plans content strategy and manages IGI’s social media, illustration, and translation.