CRISPR & Ethics
Introduction
HOW CAN WE USE CRISPR RESPONSIBLY?
The ability to edit the genomes of plants and animals raises ethical questions — some old, some brand new. How do we decide what is a good use of a transformative technology like CRISPR? Who makes the decision? How do we ensure safety and equitable access? This chapter of CRISPRpedia explores these questions and more as we think about the ethics of editing the code of life.
CRISPR ethics
What is ethics and how does it apply to genome editing?
VOCABULARY
Ethics
DEFINING ETHICS
Ethics is the practice and examination of our standards for morality — what is right or wrong — as well as broader questioning of what we owe each other and ourselves, and what we consider a good human life. Ethical judgments are influenced by culture, religion, life experience, personal values, and more. CRISPR is a technology that has the potential to be used in many different ways, for different reasons, and in almost any kind of living organism, and its use raises many ethical questions.
While CRISPR genome editing is still a new technology, the ethical issues it raises are in part shared with earlier technologies that impact health, agriculture, and the environment. What has changed is that concepts that were once theoretical, e.g. the ability to repair a harmful mutation in a gene, are now possible — or will be soon. Ethical questions become more pressing as the technology advances.
Ethical decision making is often challenging. It involves weighing competing factors that often cannot be easily measured, for example the risks or benefits of action or inaction in the long-run, or whether permitting a broadly acceptable use of a technology might open the door to other uses that would be more controversial. In this chapter, we survey some of the ethical issues that span uses of genome editing, and then dive into some of the key questions and concerns that are specific to the the use of CRISPR in human health, agriculture, and the environment.
Cross-cutting concerns
Ethical considerations for all uses of genome editing
VOCABULARY
Off-target effects, on-target effects, biodiversity, somatic cells, germline cells, eugenics, prevention, enhancement, treatment, xenotransplantation, zoonotic diseases
COMMON ETHICAL CONCERNS IN CRISPR EDITING
While each use-case of CRISPR raises specific questions, there are a number of fundamental ethical concerns that arise across all applications of genome editing. These include:
- Safety & unintended outcomes
- Biodiversity
- Access, justice & human rights
- Naturalness & relationship to nature
- Decision making & “playing God”
SAFETY & UNINTENDED OUTCOMES
A common ethical concern is whether CRISPR-based technologies are safe to use. Like all technologies, there are potential risks, potential benefits, and potential unintended effects. On a molecular level, unintended effects generally occur in two categories: off-target effects and on-target effects. Off-target effects refer to changes in the genome at a location other than the one specified by the guide RNA. On-target effects refer to unwanted changes at the site specified by the guide RNA. Both off-target and on-target effects have the potential to harm the health of an edited organism or produce unwanted phenotypes, but they can also have no noticeable effect at all.
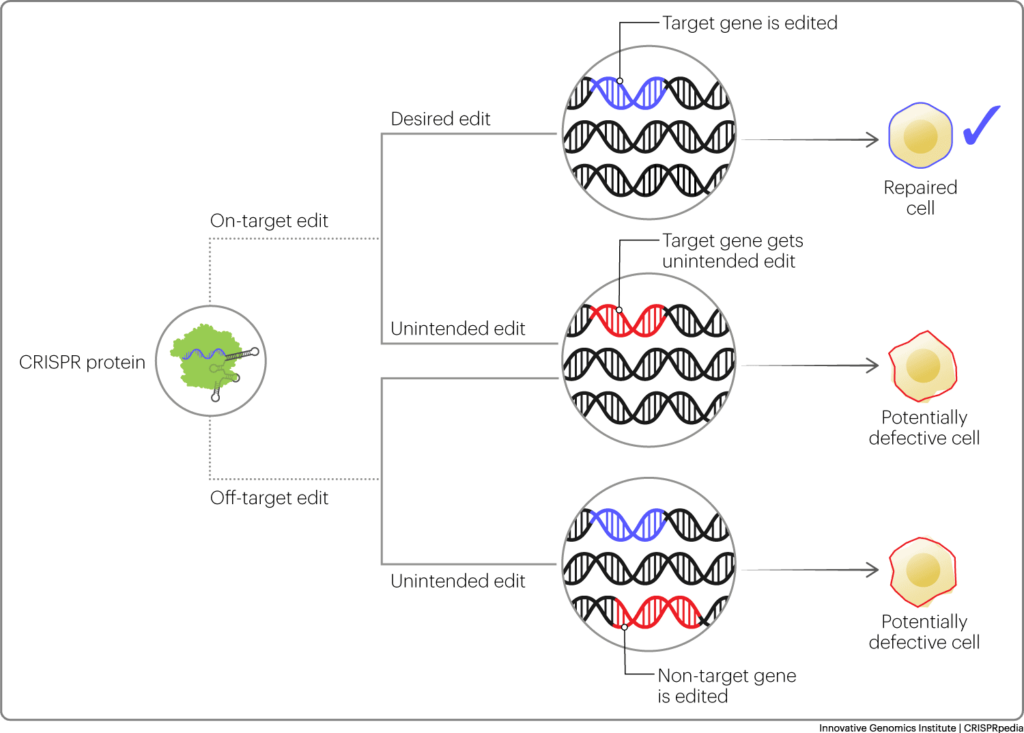
On a macro level, unintended consequences can also refer to uncertainty regarding how the use of CRISPR in humans, non-human animals, plants, and the environment may lead to hard-to-predict changes in society, culture, animal welfare, and the natural world. These effects could be beneficial or harmful. When do the potential benefits justify the risk of unintended or unwanted outcomes? In some cases, there are available alternatives, for example avoiding passing on genetic disorders can often be done through genetic screening of embryos or using donated germ cells, rather than genome editing. (These technologies, of course, raise their own ethical questions.) Improving crops can sometimes be done through selective breeding. On the other hand, genome editing allows for a greater variety of changes to be done on a faster timescale.
When regulators were faced with the decision of whether to approve the first CRISPR-based therapy for sickle cell disease, Casgevy, they had to weigh the potential benefits to patients and society against the potential risks. Sickle cell disease can cause severe pain, significant disability, and reduced lifespan, and at the time the Casgevy clinical trial began, the treatment options for sickle cell had limited efficacy.
A first-hand account of participating in a CRISPR clinical trial
Listen here: NPR interviews Victoria Gray, the first CRISPR sickle cell trial participant
The factor that distinguished this decision from any other drug approval was that no other CRISPR-based medicine had ever been approved, so predicting long-term risks involved significant uncertainty. The data so far indicate that Casgevy is a functional cure for sickle cell disease. The potential benefit of Casgevy was very high. Ultimately, regulators from the FDA and other similar bodies around the world decided that the potential benefit outweighs the safety risks and approved the therapy. Researchers continue to refine these technologies to reduce the frequency of unwanted effects, and are taking in information from early clinical trials. Whether CRISPR is safe enough for a particular application is ultimately an ethical, technical, and regulatory question that involves the severity of the condition, the potential for benefit, other treatment options, and acceptable risk level.
BIODIVERSITY
Diversity in the biological sense, or biodiversity, refers to the presence of a wide variety of different types, which includes a variety of species as well as genetic diversity within a single species.
It is widely accepted that species biodiversity is crucial to the health and resiliency of natural ecosystems. Genetic diversity within a given species is important for long-term survival of the species and resiliency to threats presented by pathogens, climate change, and more.
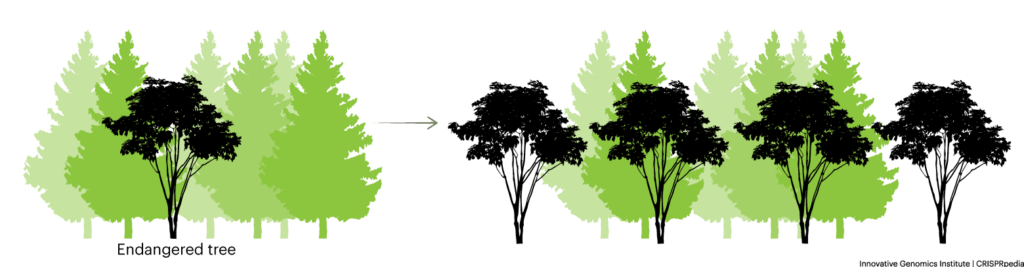
In agriculture and natural ecosystems, the threats to biodiversity include monoculture, reduced genetic variation within populations, and reduced numbers of species grown in agriculture or in natural ecosystems. It is widely accepted that monoculture practices threaten food security and one concern is that CRISPR-based tools would be used to further monoculture practices and other farming practices that threaten biodiversity. On the other hand, CRISPR-based tools could be used for purposes beyond industrial agriculture: they could be used to study genetic diversity and have been proposed as a way to preserve threatened species, combat invasive species, allow locally significant crop varieties to adapt to climate change, and even restore extinct species.
ACCESS, JUSTICE & HUMAN RIGHTS
Inequality Between and Within Countries
How quickly technologies are adopted and how widely they are used is profoundly shaped by the income of a country as a whole and of its citizens individually. Like other advanced technologies, CRISPR is being used first and most widely in wealthy, Western countries. Within those countries, genomic medicines can cost upwards of $2 million per patient. These treatments are often denied coverage by public health bodies and private insurance, limiting their access to the most wealthy.
Considerations of justice raise the concern about whether people will have access to profound technological advances regardless of country of origin or personal wealth status.
Revolutionary but costly new technology like CRISPR has the potential to worsen inequalities between rich and poor countries, as well as the potential to worsen inequalities between the rich and poor within a particular society. Wealth is the biggest factor in social determinants of health like access to housing, clean water, nutritious food, and preventative medical care. Research shows gaps in myriad health outcomes, including life expectancy, based on personal wealth level.
Right now, CRISPR is being tested to treat diseases in somatic cells — changes that will not be passed down genetically. But it could be used to create changes in sperm, eggs, or embryos — germline cells — that would be passed down through a family. We can imagine a scenario where the wealthiest people have access to germline editing to not only eliminate genetic diseases like Huntington’s disease or familial Alzheimer’s, but also reduce the risk of common conditions like heart disease, cancer, and diabetes. This could provide a great benefit to those who can access it, but the disparities in wealth and health that already exists in most societies across the world could be massively intensified and passed down at the genetic level.
Risks to Vulnerable Populations
Most ethical and religious viewpoints posit that all human beings have intrinsic value. If each human has intrinsic worth, it follows that human diversity, in regards to race, sex, gender expression, interests, abilities, and talents, likewise has intrinsic worth. There is concern that use of CRISPR to treat or eliminate diseases and disabilities could result in a less diverse, rich, and resilient culture and decrease resources for, or increase discrimination against, remaining individuals with diseases or disabilities.
To some, an engineering or an “optimization” approach to the human genome is inherently offensive to the idea of intrinsic human value or honoring diversity. Some disability rights advocates have raised concerns about CRISPR being used as a form of eugenics to eliminate kinds of humans considered less worthy or to decimate disability/difference-related subcultures like Deaf culture and dwarf culture.
Other people believe that the potential to reduce suffering, impairment, or premature death is a moral imperative and that supporting diversity should not mean forcing people to suffer unnecessarily.
NATURALNESS & RELATIONSHIP TO NATURE
CRISPR and other genomic technologies allow humans to influence our own health, the biology of animals, plants, and other organisms, and the environment, and to wield this influence at a level of precision that is unprecedented. Some people have raised concerns over the “naturalness” of these technologies. Naturalness can mean naturally occurring without human intervention, and is sometimes used in contrast to the technical, synthetic, artificial, or human-made.
Concerns about naturalness are often raised in response to medical, agricultural, and other technological innovations that affect our health and our food. Commonly, what an individual has experienced as “normal” is what they see as natural. Organ transplantation and assisted reproductive technologies, now largely viewed as acceptable and commonplace, faced concerns about naturalness when they were first introduced to the public.
Naturalness can also refer to connection with ideas of “Mother Nature” or similar cosmological views. For some, any use of CRISPR or other genomic technologies alienates humans from the natural and/or spiritual world. Some people, including some indigenous communities, are particularly concerned that humans will use CRISPR to exploit nature and harm animals and the environment, rather than be in a position of stewardship or custodianship with regards to the natural world. On the other hand, there are researchers interested in using CRISPR to protect threatened species and biodiversity and reduce human land impacts.
DECISION MAKING & “PLAYING GOD”
Concerns about the relationships between humans and the greater forces of nature, the supernatural, or the divine are central to ethics questions around whether and/or when the use of CRISPR and other genomic technologies is “playing God.” From a religious perspective, this can be quite literal: humans exercising a level of power that only God should have or changing divinely-created nature. Related secular concerns center on exercising power that we cannot fully foresee the consequences of and human arrogance in relationship to nature.
Of course, not all people share these religious or spiritual worldviews. For some, CRISPR is a tool that enables us to do good in new ways — in some cases, a technology that there is an ethical imperative to use.
These competing views raise the question of how decisions about the use of new technology should be made. Who decides? Who should get to decide? What processes can ensure input from diverse stakeholders?
In addition to the ethical issues mentioned above, the potential to edit the human genome raises questions around appropriate use of editing (somatic versus germline editing, treatment versus prevention versus enhancement), reproductive autonomy and the autonomy of the child/fetus, unequal access, and societal effects. In the following sections, we will look at each issue more closely.
Good genes?
The term “eugenics,” coined by Francis Galton in the 1880s, comes from the ancient Greek words eu, which means “good,” and genos, which means “birth.” Galvanized by emerging evolutionary theory, Galton suggested that certain individuals — and white Europeans, more generally — have superior genes which lead to superior intelligence, moral character, and other desirable traits. He believed that selective breeding of humans could lead to smarter and healthier human populations and eliminate social ills. By the 1920s, political and thought leaders in countries around the world began to promote ideas and policies meant to encourage reproduction by some members of the population and stop reproduction by others. These practices were based in common white supremacist, ableist, and other prejudiced beliefs.
Eugenicists in the United States believed that the number of healthy, white people was decreasing, and that genetically undesirable people were reproducing at higher rates. To reverse this, they promoted institutionalization and involuntary sterilization of members of certain populations, including people with physical or intellectual disabilities, people with mental illnesses, poor people, sexually promiscuous women, LGBTQ+ people, and prisoners. Between 1907 in 1960, over 70,000 people in the United States were subject to legal involuntary sterilization. Black, Hispanic, and Native American people were disproportionately impacted by these practices, with at least 25% of Native American women of reproductive age being subject to forced sterilization in the 1970s alone. While many of these laws were overturned in the late 1970s, some of these practices continued for decades. In California, state laws allowing for involuntary sterilization of prison inmates were only overturned in 2010.
The eugenics movement informed the Third Reich in Nazi Germany, which forcibly sterilized over 400,000 people. Nazis killed over 6 million Jews, as well as individuals from other groups deemed inferior, including ethnic minorities such as Roma and Serbs, people with disabilities, and people convicted of committing crimes. Eugenics was used to justify not only the violation of rights to basic bodily and reproductive autonomy, but also ethnic cleansing and genocide.
Many ethicists and individuals from minoritized groups are concerned about the potential to use genome editing as a tool for eugenics. Some see genome editing to prevent severe diseases or disabilities as a slippery slope to a new eugenics movement or a society with a genetic upper class and a genetic lower class, as in the movie Gattaca. These concerns need to be front-of-mind when crafting policies, and individuals from historically marginalized groups should be key voices in decision-making.
Learning resources:
- Eugenics and Scientific Racism — from the National Human Genome Research Institute
- Unwanted Sterilization and Eugenics Programs in the United States — from PBS
Somatic- vs. germline-cell editing
Contrasting changes that only affect an individual and changes that are heritable
VOCABULARY
Somatic cell, germline cell
CONTROVERSIES & CONSENSUS
As we’ve seen with the success of Casgevy for sickle cell disease, CRISPR has the potential to treat or even cure genetic diseases. While there is broad consensus in favor of using CRISPR for somatic cell editing to treat serious illnesses, editing human germ cells — eggs, sperm, or embryos — is highly controversial and largely prohibited by regulators. Most scientists in the field agree that CRISPR technology is not yet precise enough for there to be any ethical use of germline editing, and there are often alternatives. Many also feel that we do not yet understand the potential consequences, uses, or misuses of the technology well enough to use it for germline editing.
Somatic cells are all the cells of the body — like skin cells, bone cells, and muscles cells — that do not contribute to the genomes of offspring. Any changes to the genomes of these cells, including random mutations, are not passed on to the next generation. Germline cells — eggs, sperm, and embryos — are the cells that do contribute to the genomes of offspring. Changes to the genomes of these cells may be passed on to the next generation.
Despite this broad consensus, in November 2018, Chinese scientist He Jiankui announced the birth of twin girls resulting from embryos that he had used CRISPR to edit in an in vitro fertilization process. A third baby was born in 2019. According to He, the edit to the gene CCR5 was intended to protect the girls from HIV infection because of their fathers’ HIV-positive status — although the well-known technique of sperm washing reduces the chance of transmission to less than 1%. The response to He’s announcement was international outcry and condemnation, and he served three years in prison. He’s actions triggered calls for clear and strict guidelines on the use of CRISPR in human embryos and improved methods for research oversight.
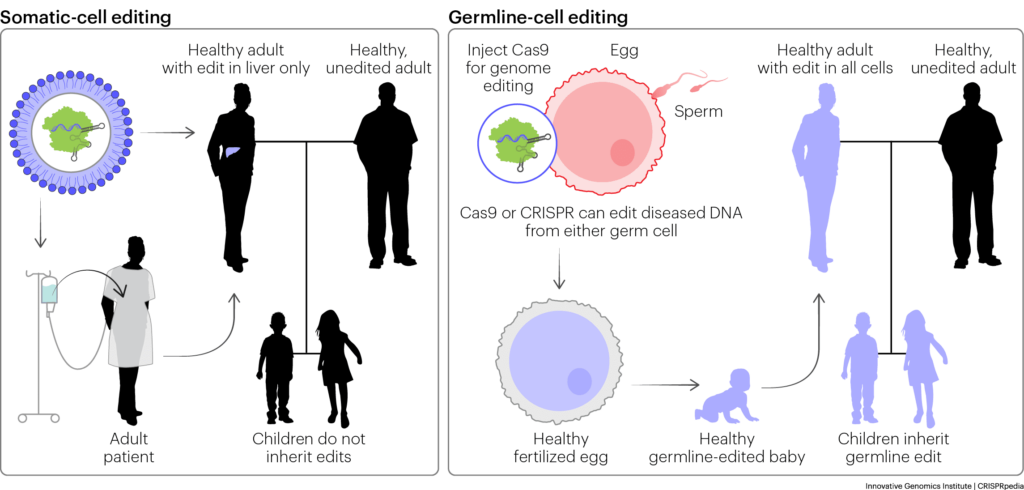
As scientists continue to refine genome-editing technologies, the ethical and regulatory questions around germline editing become more urgent. Germline editing raises unique ethical questions because any changes to the genome can be passed down to an individual’s biological children. This could mean freeing a family from hereditary disease — but it could also mean passing on unintended and potentially harmful edits. Related to the “playing God” category of concerns, some people feel it is profoundly hubristic to make edits that can be transmitted. As explored above, there is also the risk of worsening societal inequality if only wealthy individuals have access to this technology. There are also concerns that germline genome editing could facilitate the return of eugenics.
Treatment, prevention & enhancement
Medical ethicists make a distinction between treatment and prevention versus enhancement. Treatment and prevention are meant to address a disease or disability, while enhancement refers to optional augmentation of a medically “normal” trait or choosing a trait like eye color.
As mentioned above, there is broad support for editing somatic cells to treat serious illnesses like sickle cell disease or cancer. In these cases, many people agree that the potential benefit to the patient might outweigh the potential risk.
The more open questions center around using CRISPR to prevent diseases or disabilities, or for genetic enhancement of a desired trait.
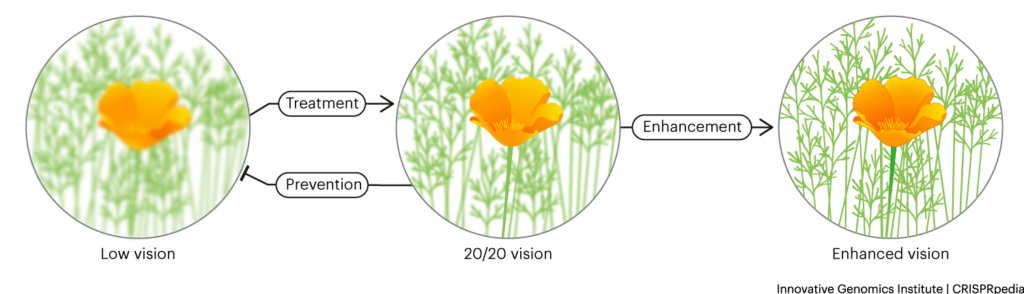
PREVENTING DISEASE & DISABILITY
The ethical questions around disease prevention mainly center around risk versus benefit profile. For someone who is already suffering from a serious illness, the benefit is obvious. But what if someone has a genetic variant that will cause them to develop a difficult condition later in life? We can imagine a scenario where a healthy young adult might have a choice about whether to take a treatment that would prevent the genetic disease, but could face a risk of side effects that would affect their immediate well-being. What if an individual has a genetic variant that increases their likelihood of developing a condition like heart disease, but doesn’t guarantee it? Could preventative treatment be warranted in that case? These questions are open for discussion.
Diversity and inclusion concerns are a major ethical issue in disease and disability prevention. Would genetic treatment to eliminate certain diseases and disabilities stigmatize people living with similar conditions? Would the level of care and support for sick and disabled people decrease? Would society as a whole become less compassionate, or suffer because of the reduced diversity of bodies and experiences? We will explore these questions more in a later section.
Another frequently cited concern is that editing for disease and disability will lead to editing for enhancement. This is an example of a slippery-slope argument where a claim is made that one decision or course of action will inevitably lead to more extreme courses of action.
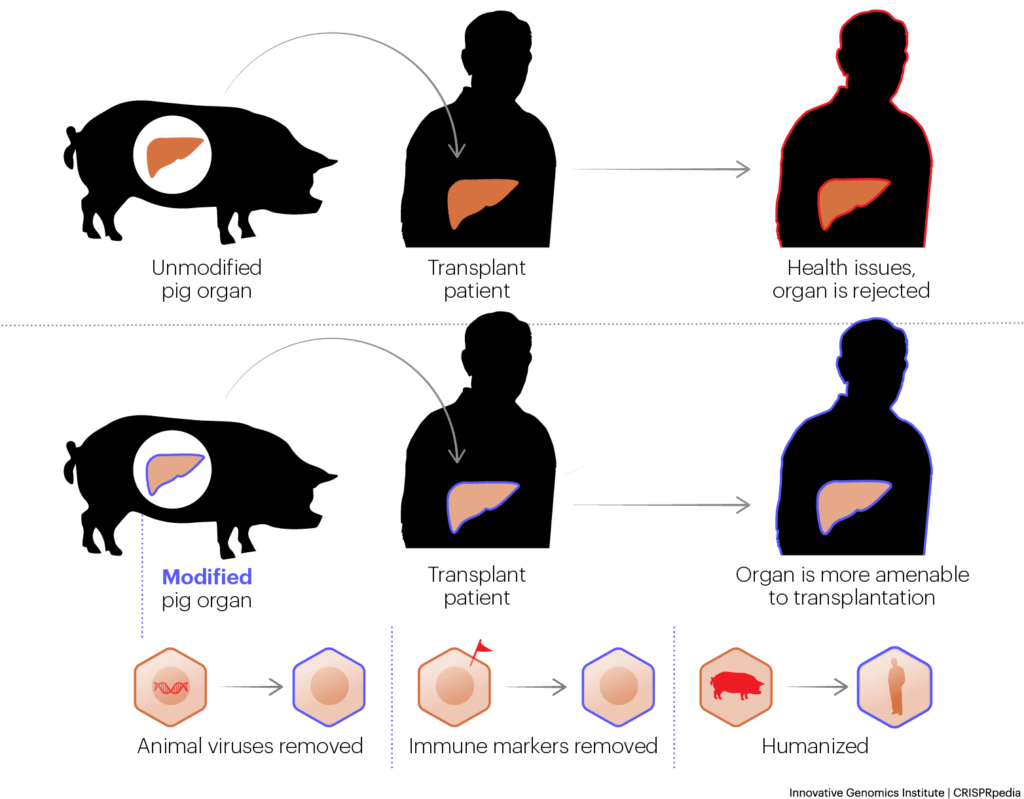
XENOTRANSPLANTATION
Because there is a perpetual shortage of donor organs, xenotransplantation— the transplantation of organs from animals to humans — has long been of interest and raises distinct ethical issues. Pigs are generally considered to be the most suitable potential donor animal because of comparable organ size and ease of husbandry. Human immune reactions and the potential spread of zoonotic diseases have been the biggest barriers to the practice. The potential of CRISPR genome editing to reduce these risks has revived interest in xenotransplantation. A related possibility is growing human organs from human stem cells in animal fetuses. In addition to animal welfare concerns, questions about naturalness, access, and equity are brought up by the potential of xenotransplantation.
ENHANCEMENT
Enhancement refers to using genome-editing tools to increase desired traits like intelligence, musical ability, or choosing traits like height or skin color. Enhancement is usually discussed in the context of germline-cell editing, since many traits are developmentally determined and could not be induced with editing later.
Enhancement is largely considered unethical by scientific leaders and world governments. For the most part, it’s not something that we have the technical ability to do yet, and we may never have the ability to reliably affect certain traits that are the product of the interaction of many genes and the environment, like intellectual or personality traits. Still, if pop culture is any measure, there is a fascination with the idea of choosing and enhancing human characteristics.
The idea of enhancement raises several ethical concerns that we have touched upon:
- Naturalness and “playing God”: Some people believe that only God or chance should determine someone’s genetic makeup. Others might argue that enhancement is in the realm of reproductive autonomy of parents, or could lead to a society with healthier, more talented, and more productive members.
- Unintended consequences: In addition to unintended effects at the genetic level, there is the concern about whether someone who is genetically imbued with, for example, musical ability might be shut off from their other interests, passions, and talents.
- Diversity: Enhancement might lead to the loss of human genetic diversity and human trait diversity. In addition to concerns explored above, it is well-established that genetic diversity of a population is crucial to the long-term health and survival of that population.
- Inequality concerns: Unequal access to, or willingness to use, genetic enhancement technologies could lead to the development of a society with a genetic lower class. This concern is also expressed at the global level, with the idea that rich countries could enhance their economic advantage with these technologies.
- Reproductive autonomy: Another set of concerns revolves around the reproductive autonomy of parents versus the autonomy of the potential child. Some people argue that parents should have the right to make choices about genome editing their embryos, fetuses, or newborns in the same way that parents have the right to make other medical decisions for their child. Others argue that genome editing is fundamentally different from a medical treatment that the child wouldn’t pass down to their own children, and violates the future child’s autonomy.
Case studies in disability & diversity
It is easy to understand the use of genome editing for an extremely painful and life-limiting condition like sickle cell disease, or disease that causes death in early childhood like Tay-Sachs disease. But what about genetic conditions that individuals can live with and still have a high quality of life? Let’s explore two case studies: prenatal screening for Down syndrome and hearing-restoration treatments for individuals who are deaf or hard of hearing (HOH).
DOWN SYNDROME
A real-world case study in reproductive autonomy is the widespread use of prenatal screening for Down syndrome in Denmark. Down syndrome is a genetic condition that occurs when an individual has an extra copy of chromosome 21. Down syndrome typically leads to intellectual disability and specific health risks, particularly to the heart. Individuals with Down syndrome are generally friendly, affectionate, with a happy and social disposition, and can have a high quality of life with the right support.
In 2004, Denmark became one of the first countries in the world to offer free prenatal screening for Down syndrome to all pregnant people. The rate of pregnancy termination when tests indicate Down syndrome in Denmark is close to 99%. Within a year of implementing the program, the number of children born with Down syndrome in Denmark was halved, at around 30 babies per year. Some people argue that this has led to a reduction in visibility, acceptance of, and support for people with Down syndrome, and judgment against parents who have children with disabilities that are considered avoidable. Bioethicist Rosemarie Garland-Thomson refers to this kind of prenatal screening and pregnancy termination as “velvet eugenics.” Others argue that prenatal screening is crucial to allow parents to make informed choices around family planning and that the overall reduction of the level of disability within a society is beneficial.
DEAFNESS AND HEARING LOSS
In 1990, the US FDA approved the use of cochlear implants (CIs) to restore hearing in children as young as two-years old. Their use has been controversial since then. Many deaf people in the United States are part of the rich subculture of the Deaf community, with its own history, customs, traditions, and language (American sign language). Many individuals in the Deaf community consider themselves to have a difference, but not a disability.
deaf vs. Deaf
Lowercase d “deaf” is used to refer to individuals with severe or profound hearing loss. It is a physical descriptor. Capital d “Deaf” is used by some deaf people to denote embracing Deafness as a cultural identity. This distinction underscores the challenge of deciding whether hearing loss should be viewed as a disability to be prevented or corrected, or simply a difference.
Some members of the Deaf community see CIs as a direct threat to the existence of their community, their culture, and the identity of Deaf individuals. From this perspective, the use of CIs can be seen as a sort of eugenics that doesn’t eliminate individuals, but eliminates a core aspect of their experience or identity. Another complaint is that CIs do not restore normal hearing: they restore a limited range and resolution of sound, which may allow some individuals to move more easily in the hearing world, but can also be difficult and exhausting for individuals with CIs. More recently, a study published in The Lancet used gene therapy to restore some level of hearing in children with profound genetic deafness. While the technology is different, it raises the same questions about community, identity, and how disability is defined.
Ethics & editing in agriculture
Using CRISPR in plants and animals
VOCABULARY
Mutation breeding, transgenesis/GMO, staple crops, biodiversity, technology escape, moral hazard
THE GOALS OF GENOME EDITING IN PLANT AND ANIMAL AGRICULTURE
Genome editing is becoming more common in both plant and animal agriculture. In large part, the goals are the same as selective breeding practices that have been used for thousands of years, as well as other kinds of genomic technology that has been used more recently: to increase yields and improve taste. More recent objectives also include reducing the need for weeding, reducing the use of pesticides and fertilizers in plant agriculture, increasing resistance to plant and animal pathogens, improving consumer traits like nutrition, reducing food waste, and adapting both crop plants and farmed animals to climate change.
Until the advent of CRISPR, animal agriculture largely relied on selective breeding. In crop plants, however, plant breeders have used mutation breeding, transgenesis, and, more recently, genomics-assisted breeding to develop improved crops. Mutation breeding, which involves the deliberate creation of mutations using radiation or chemicals with the goal of finding a beneficial trait, has received little press and broad acceptance. Transgenesis, in which foreign genes are inserted to introduce a new trait resulting in what is commonly known as a “GMO” crop, has been more controversial and is most commonly used for two purposes: to give crop plants resistance to chemical herbicides, so farmers can “weed” fields by applying herbicides, and to make crop plants toxic to insect pests without the use of chemical insecticides. Genomics-assisted breeding techniques use the information provided by genome sequencing to help select parents to cross-breed to create unique combinations of desirable traits.
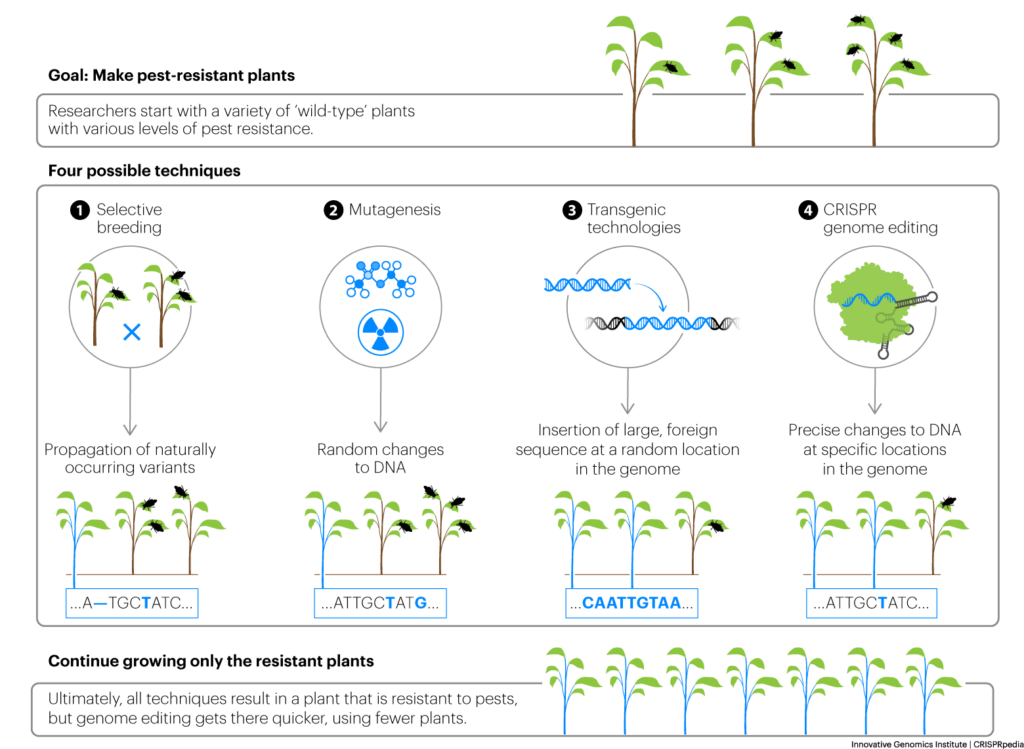
CRISPR genome editing is in this lineage of tools that have the shared goal of improving agriculture. Each of these approaches, including selective breeding, alters the genome — but CRISPR does it with unprecedented precision, creating new possibilities or accelerating the breeding process. These new possibilities come with ethical questions, some of which are concerns expressed towards previous technologies and some of which are specific to genome editing. These questions include issues around biodiversity, the possibility for edited plants to unintentionally mix with wild or unedited populations (technology escape), animal welfare, plant seed conglomerates and access to edited seeds, addressing climate change, and the potential to benefit farmers around the globe.
FEEDING A HOT, HUNGRY WORLD
Ethical questions are sometimes framed in consequentialist terms as a balance between the risks and benefits of a new technology. In health, these decisions are often at the individual scale. In agriculture, the effect is often at a much greater scale.
For example, over 800 million people in the world are undernourished. Many of them don’t get enough protein or calories in their diets. Genome editing could help feed the hungry in a few ways:
- Growing more food with fewer resources: Many crops require very specific growth conditions. Farmers can only grow them in certain climates during particular times of the year. Alternatively, farmers can force growth using a lot of water, fertilizer, and other resources to force them to grow off-season. Genome editing could be used to create crops that grow better under a wider variety of conditions and create higher yield, and can help local varieties be more resilient to a changing climate.
- Making it easier to harvest or process crops: Some crops have qualities that make them difficult or expensive to harvest or process. This could include fruit that needs to be picked by hand or cassava, which needs to be heavily processed to remove toxic compounds. Genome editing could be used to alter crops to make them easier to harvest or process, making it easier for people to use them for nutrition.
- Decreasing food waste: Every year we waste over 1 billion tons of food. This waste happens before foods leave the farm, as well as in stores and in our homes. Researchers are using genome editing to make foods last longer, for example, creating mushrooms that don’t brown as quickly.
- Improving nutrition: Around 3 billion people worldwide lack access to a healthful diet. Micronutrient deficiencies are common in the developing world, occurring in places where people lack access to diverse food sources and therefore don’t get the appropriate vitamins and minerals from different kinds of food. Researchers can use genome editing to improve the nutritional properties of staple crops. Strategies have involved increasing the levels of nutrients (e.g., anthocyanins and Vitamin D in tomatoes) or reducing toxins (e.g., cyanide precursors in cassava).
Adding to the issue of hunger, the global food supply is under threat from climate change. These threats are multiple: increased spread of pathogens and pests, extreme temperatures, drought and flooding, and increased water salinity are some of the greatest concerns.
These risks are expected to inflict the greatest damage on low- and middle-income countries. Researchers are using CRISPR to try to create variants of staple crops like rice, wheat, and sorghum, which provide a large percentage of a population’s calories and nutrients, as well as livestock, that can withstand pathogens and environmental extremes. Many feel an ethical imperative to use all the tools at hand — including genome-editing — to prevent devastating famines.
Gene editing, GMOs, and “naturalness”
As detailed in the first section of this chapter, for many people, genome editing raises concerns about naturalness. This issue is important not only for public opinion but also regulation. The US and European Union regulate transgenic — commonly referred to as GMO — organisms differently than organisms that are not transgenic.
Genome-editing technologies like CRISPR can be used to create the kinds of genetic changes that can occur in nature (e.g., simple changes to existing genes) or to create transgenic changes (insertion of a gene from a different source). In the US and European Union, if a genome-edited crop plant or agricultural animal has a change that could have occurred in nature, it is under no particular regulatory requirements relative to conventional plants or animals. If it is transgenic (GMO), regulations are much stricter. While research has shown no evidence that transgenic organisms are inherently harmful to ecological systems or that consuming them harms human health, many people express ecological and health concerns. And many people feel transgenesis is unacceptable because it is “unnatural” in that the changes could not have occurred without human intervention.
BIODIVERSITY
Biodiversity can refer to diversity at different levels: one might be at the level of an ecosystem and refer to the number of different species present. Another might be at the level of a population group of a specific species in a particular location, and refer to the diversity of the gene pool of that group. It is widely accepted that more biodiverse ecosystems are more stable and resilient, and more genetic diversity within a population group confers greater average health and greater group resilience to pathogens, environmental threats, and more. Concerns around biodiversity relate to not just diversity as a value, but also to the risks of unintended consequences and of threatening “naturalness” by potentially affecting natural populations.
Genome-editing technology could be used to protect or restore biodiversity. Genomic technologies have already been used to create plants that are unappetizing or toxic to insect pests, removing the need to spray crops with toxic insecticides that can harm a variety of insects, small vertebrates, and pollute water sources. Plants with reduced need for chemical fertilizers also help protect the health of waterways and the organisms that depend on them. Genome editing could also potentially be used to protect biodiversity by providing pathogen resistance to native species under threat or preparing native species to better withstand climate change. Some people are even interested in “de-extinction” – using CRISPR to restore extinct species, though this is technically challenging and raises many questions. These include questions about best strategies and appropriate resource usage to preserve or restore natural ecosystems, and which species should be restored given that over 99% of species have gone extinct over evolutionary history.
Market forces often drive the application of technologies towards financial gain and consumer demand. In agriculture, this may mean creating one or a few particularly desirable strains of a crop plant or agricultural animal at the cost of diversity in the population’s gene pool. This could create a vulnerability in the food system in the case of a pathogen or other threat. Threats to biodiversity can also include threats to culturally significant plants and animals. These are risks of industrial agriculture in general, but may be intensified by the use of genome-editing technologies.
TECHNOLOGY ESCAPE
A significant ethical concern, related to biodiversity, is the potential for technology escape. Technology escape is the unintentional mixing between genome-edited plants and conventional (unedited) or wild populations. This is a concern that is shared with all earlier forms of plant breeding, but is the most acute in transgenic crops where a foreign gene might escape into other populations. Because of the nature of plant versus animal reproduction, this concern primarily applies to plants where pollen can be carried out of defined zones by pollinators like insects or bats, or seeds can unintentionally migrate across farm lines. This issue is primarily mitigated by distance: crop plants are rarely grown adjacent to their wild relatives. In the small number of documented cases of technology escape from transgenic crops, the traits that are desirable for farming do not adapt them for success in wild environments and they don’t tend to spread rapidly. Some organisms, particularly bacteria, are capable of taking up DNA from their environment. It’s hard to know what effects — if any — this “gene flow” could have.
The biggest risk of technology escape is the potential for negative impact on biodiversity, although this has not yet been observed. The possibility of technology escape brings up multiple potential regulatory and ethical issues. One is the question of intellectual property: if certain genetic changes to plants are patented, what risks are incurred by farmers who are inadvertently growing them through technology escape? And who is liable if technology escape causes harm to a natural environment or another farm?
Another concern centers around consent. Farmers may choose to grow particular genome-edited varieties or cultivars. But if these edited organisms escape into the broader environment, that has the potential for broad impact and brings up the question of citizen consent to genome editing of common property.
ANIMAL WELFARE
Industrial animal agriculture has many critics who are concerned about the environmental impact, animal welfare, and more. Genome editing raises new ethical concerns and enhances the urgency of existing ones. One concern is the small amount of space per animal for industrial or “factory” farmed animals in the United States. Some limits on crowding are imposed in part by concern for disease: as in humans, overcrowding of farm animals is a major risk factor for spreading communicable diseases. Researchers are actively interested in creating genome-edited animals that will be resistant to common communicable diseases or maintain the health of the animal in other ways. In the instance of communicable disease, critics argue that humane treatment of farmed animals is sufficient dramatically limit this risk and editing to make them more resilient could lead to even more overcrowding.
CONCENTRATION OF POWER & LIMITED ACCESS
Because developing genetically modified crops has historically been expensive, time-consuming, and heavily regulated, their acute economic benefits became concentrated in the few large companies that could afford to create such crops and the large-scale farmers who could afford the high-priced seeds. With the intellectual property, power, and wealth in the hands of few large agribusinesses, this created unequal access and intensified global wealth inequality.
CRISPR-based genome editing has the potential to make it easier, faster, and cheaper to create crops with beneficial traits. This has changed the market already, making it possible for small start-up companies to create new crops and opening the potential for academic and government-funded researchers to have a greater impact, including at the local and regional level. This could lead to broader access and broader economic benefit. Because this field is still developing, questions remain about access to edited crops.
Slick-coat cattle & moral hazards
Increasing temperatures don’t just affect humans: they can also be a major problem for livestock. Warming temperatures cause physiological stress to cattle. Their body temperature and breathing rate increase, and in dairy cows, milk production goes down.
One recent application of genome editing is the development of “slick coat” cattle that recreates a shorter coat trait that helps keep cattle cooler in hot climates. The changes made by CRISPR-based gene editing resemble naturally occurring variations in the same gene often found in tropical cattle breeds. Gene editing enables the transfer of this short-haired trait into good meat-producing breeds in a streamlined manner. In a historic decision in 2022, the United States FDA made the determination that a genome-edited beef cattle with a short coat can be commercialized — the first time the FDA has given a green light on a gene-edited animal intended for human consumption.
Proponents of slick-coat cattle argue that these modifications are in the best interest of the animal and promote animal welfare, while also maintaining the productivity of animal agriculture in climate extremes. Their critics worry that these types of applications create a moral hazardwhereby the availability of the technology unintentionally reinforces the behavior it is trying to address. Cattle farming is responsible for a significant portion of global methane emissions, so some argue that the appropriate decision for a warming world is to reduce their use in agriculture, rather than find ways to pasture them in hotter temperatures and continue to add to global methane burden.

Ethics & environmental applications
Using CRISPR for fighting climate change, restoring the environment, and more
VOCABULARY
Carbon capture, green manufacturing, bioremediation, biosensing
GOALS OF GENOME EDITING FOR THE ENVIRONMENT & CLIMATE
There is growing interest in adapting genome editing for environmental applications. This research is largely preliminary but some of the goals include:
- Ecosystem protection: Genome editing could be used to make native species more resistant to pathogens and climate change. Invasive species can wreak havoc on local ecosystems. Gene drives could be introduced into invasive species and function as a kind of genetic birth control to reduce their numbers or even eliminate the population, restoring the health of the local environment.
- Reducing pollution: Genome editing is being used to make plants resistant to pests and pathogens, reducing or eliminating the need for chemical pesticides, fungicides, etc., which can pollute soil and local water sources. Genome editing of microbes could enhance nitrogen fixation, reducing or eliminating the need for nitrogen fertilizers which, like chemical pesticides, can pollute water sources.
- Mitigating greenhouse gas emissions: Cattle raised for meat and milk are a major source of methane, a highly potent greenhouse gas. Researchers are working on ways to reduce methane emissions from cattle, including using genome editing to create grass that is easier to digest and editing the microbiome to reduce methanogenic activity. Another major source of methane emissions is rice cultivation. Certain microbes (archaea) that thrive in the anaerobic environment of soils below flooded rice paddies generate methane, and researchers are investigating ways of reducing or eliminating this impact.
- Increasing carbon capture and storage: Plants and microbes have evolved ways to capture atmospheric carbon at a global scale, but their work isn’t sufficient to capture all of the excess carbon generated by human activity. Researchers are investigating ways of using genome editing to improve the efficiency of carbon capture in both agricultural crops and forests, as well as ways of ensuring that the carbon remains stored in soils.
- Green manufacturing: Plastic pollution is an enormous and growing problem. Genome editing could be used to make yeast, E. coli, or other organisms produce compostable plastic-like materials that could reduce plastic waste, or reduce pollution associated with the manufacturing of various organic compounds used in industry.
- Enhancing bioremediation: Microbes are sometimes used for cleaning up contaminants like oil from oil spills. Genome editing could be used to create microorganisms with increased capacity to signal contamination (“biosensing”) and bioremediation.
The ethical questions raised by environmental applications of genome editing are similar to those raised by agriculture. They center around issues of consent for use in the natural environment, technology escape, and unintended consequences.
Regulating CRISPR ethics
VOCABULARY
Policy, gene drive
HOW DECISIONS ARE MADE
Views of ethics change over time, and vary among cultures and individuals within cultures. So how are decisions made and who gets to decide?
Even the question of who decides brings up its own questions. Should decisions be made solely by technical experts? By a combination of experts and bioethicists? By community or spiritual leaders? By the populations most likely to be affected, especially populations likely to face stigma, exclusion, or discrimination? By the general public? How do we ensure that the people making decisions understand the technology they are evaluating?
CRISPR POLICY
Policy refers to how the use of CRISPR is regulated in and outside of labs. Policies vary by country and are dynamic, changing over time. Policies are often made by governmental bodies in consultation with experts or expert panels. While policies are being actively considered, engagement with the non-expert public is sometimes sought out. This kind of public engagement can take multiple forms: town halls, policy documents being shared online with the opportunity for citizens to submit comments, citizens’ juries, and more. Sometimes decisions about the use of technology are made directly by the public through voting on proposed legislation.
🎥 | Learn more about citizens’ juries in the short documentary below.
In general, there are four common ethical approaches to regulation:
- Regulation by safety assessments: Before bringing them to market, companies must do a variety of experiments to show that their products are safe. This data must show that products meet the standards set by regulatory agencies. If they do, products can be marketed and sold.
- Regulation by use of the product: Products have different safety risks depending upon how they’re used. For example, products intended for children are evaluated differently than products intended for adults because of both physical health risks and behavioral differences. Regulations can be made on the basis of intended use.
- Method and novelty-focused regulations: Products can be regulated based on the processes used to create them. Many countries distinguish between transgenic technologies and genome editing that creates changes that could occur in nature. Generally, the latter is regulated much more loosely or treated in the same manner as products developed through conventional breeding practices. Other regulators may put more scrutiny on novelty. For example, if a new transgenic plant is resistant to insect pests using an approach that has already been approved in other plants, it may not be considered novel and may not need to undergo extensive testing.
- Regulation according to societal concerns: Some regulations seek to address concerns about cultural impacts, economics, political consequences, and much more. The importance of these various societal concerns are dictated by people’s diverse values. Individual nations and jurisdictions differ in how much their regulations incorporate such societal concerns.
Regulation of genome-edited crops and medicines is an area of active development and exploration for governments around the world.
The mice against ticks project
Lyme disease is a growing problem in the United States and has long been a major public health threat in New England. Acute cases cause flu-like symptoms, fatigue, and joint pain. If not treated quickly, Lyme disease can lead to chronic health issues including arthritis and severe neurological problems. Lyme disease is caused by the bacterium Borrelia burgdorferi. White-footed mice serve as a reservoir for the bacterium.
In 2015, researchers at the Massachusetts Institute of Technology started the Mice Against Ticks project to explore using new genomic technology to limit the spread of Lyme disease by stopping white-footed mice from being a reservoir. They could do so by giving the mice DNA that encodes two types of proteins. One is an immune protein that would prevent Borrelia burgdorferi bacteria from infecting the mice. The other protein would prevent ticks from feeding on the mice.
They were interested in exploring using these mice starting on an island setting where any changes would be contained to the island. They chose the Massachusetts island of Nantucket where Lyme disease is rampant as an initial target. From the beginning of the project, the researchers decided to work in close communication with Nantucket residents. They would only move forward with the consent of the population. They presented Nantucket with two options for action: putting the anti-Lyme genes in a gene drive (see the CRISPRpedia chapter on CRISPR Technology for an in-depth explanation of gene drives) in a small number of mice who would be released to mate within the local population and spread the new genes rapidly, or using a classic genomic engineering approach, adding the DNA in a more typical fashion that would spread more slowly through the mouse population. In the second scenario, a large number of mice would be used and the expected outcome would be an initial period of reduced Lyme transmission, after which the new genetic material would slowly be lost and Lyme rates would increase again.
Researchers presented these solutions to Nantucket. After intense deliberation, the citizens decided that gene drives were too risky — if the mice got to the mainland, it could potentially spread the new DNA widely and rapidly. Researchers are now developing the classically-engineered mice. They will be testing them on an unpopulated island before releasing them on Nantucket. After testing, the Nantucket residents will have another opportunity to approve or reject the use of engineered mice on their island.
Listen to episode 7 of the Patient Zero podcast from New Hampshire Public Radio for more on this story: Listen here.

SCIENTIFIC REVIEWER
Hope Henderson holds a B.A. in Biology from Brown University and a Ph.D. in Molecular & Cell Biology from the University of California, Berkeley. She joined the IGI in 2019 to work in science communication. In addition to serving as IGI’s main writer, she plans content strategy and manages IGI’s social media, illustration, and translation.
SCIENTIFIC REVIEWER
Jodi Halpern is a Professor of Bioethics and Medical Humanities in the Joint Medical Program and the School of Public Health at the University of California, Berkeley. Her work brings together psychiatry, philosophy, affective forecasting, and decision neuroscience to elucidate how people imagine and influence their own and each other’s future health possibilities.
HOW TO CITE
Henderson HR, Ramit G, Tolpa T, Murdock AG, and Halpern J. (2024) CRISPR & Ethics. In Hochstrasser et al. (Eds.) CRISPRpedia. Innovative Genomics Institute, University of California, Berkeley. Retrieved from: https://innovativegenomics.org/crisprpedia/crispr-ethics/ (Last updated: November 21, 2024.) https://doi.org/10.60640/G2H59Q
SHARE PAGE