Establish a SARS-CoV-2 Testing Lab at Your Institution
Purpose
As our first step in responding to the COVID-19 pandemic, the IGI assembled a dedicated team that worked around the clock for three weeks to set up a CLIA-certified SARS-CoV-2 testing lab that has been testing patient samples since April 2020.
We learned a lot in the process, and want to share these lessons. We hope that many other groups will follow suit and set up their own testing labs.
Please read our open access how-to guide, linked below. If you have questions that cannot be answered in our guide, contact igi-covid19@berkeley.edu. We will do our best to respond to inquiries.
Blueprint to Establish a SARS-CoV-2 Testing Lab at Your Institution
Nature Biotechnology paper (June 18, 2020)
Contains content of preprint, plus fully-automated method validation and observations from early testing.

medRxiv preprint (April 24, 2020)
Contains description of regulatory navigation to date and semi-automated method validation. See supplementary files at the end of this page.
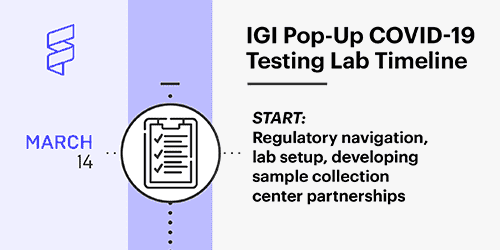
IGI pop-up COVID-19 testing lab timeline
Our timeline for establishing the testing lab.
Replicating Our Efforts With Data Management
A key factor in our quick success was the in-depth support we have received from Third Wave Analytics, Salesforce, and MuleSoft.
In a matter of weeks, Third Wave Analytics built a robust Laboratory Information Management System (LIMS) on their LockBox platform to manage the technical data flow of clinical samples in and out of the lab. Subsequently and with pro bono support from Salesforce and MuleSoft, Third Wave Analytics also built and implemented a HIPAA-compliant, secure physician portal wrapped around the technical LIMs to accept requisitions and return clinical-grade results to providers working with the facility.
Establishing this system was only possible due to a discounted framework and a herculean effort made by the tireless and unrelenting hours of Third Wave Analytics programmers and support from Salesforce.
In an effort to allow other colleagues to quickly and affordably build off of our efforts, Third Wave Analytics has agreed to release the identical framework of the IGI build for 50% off the licensing fee alone for other UCs ($4500) and a similarly nominal cost for non-UC facilities.
It should be understood that other fees will be required for customization and alterations to the IGI system to fit any variations at individual facilities. For reference, however, the IGI has spent over $100K to build what others can now access for a nominal fee. Integrations with third-party companies that provided services (Salesforce and Mulesoft) are not under the current MOU and may be needed.
Company Contacts
Third Wave Analytics: Chris Gawronski (cgawronski@thirdwaveanalytics.com)
Salesforce (required for physician portal): Tyson Read (tyson.read@salesforce.com)
MuleSoft (Incorporation into EMR systems): Sam Steiny (sam.steiny@mulesoft.com)
Internal UCB reference for questions and introductions: Shana McDevitt (shana.mcdevitt@berkeley.edu)
*Please put “Third Wave MOU” in subject line
Supplementary Files
Our earlier preprint on the testing lab blueprint is freely available on medRxiv. Supplementary files referenced in that preprint are included below. All supporting material for the Nature Biotechnology version are linked directly within that manuscript.
Related Publications
Optimizing COVID-19 control with asymptomatic surveillance testing in a university environment
Brook CE, Northrup GR, Ehrenberg AJ, Doudna JA, and Boots M. Epidemics
Robotic RNA extraction for SARS-CoV-2 surveillance using saliva samples
Hamilton JR, Stahl EC, Tsuchida CA, LinShiao E, Tsui CK, Pestal K, GildeaI HK, Witkowsky LB, Moehle EA, McDevitt SL, McElroy M, Keller A, Sylvain I, Hirsh A, Ciling A, Ehrenberg AJ, Ringeisen BR, Huberty G, Urnov FD, Giannikopoulos P, Doudna JA, and the IGI SARS-CoV-2 Consortium. PLoS One
Launching a saliva-based SARS-CoV-2 surveillance testing program on a university campus
Ehrenberg AJ, Moehle EA, Brook CE, Cate AHD, Witkowsky LB, Sachdeva R, Hirsh A, Barry K, Hamilton JR, Lin-Shiao E, McDevitt S, Valentin-Alvarado L, Letourneau KN, Hunter L, Keller A, Pestal K, Frankino PA, Murley A, Nandakumar D, Stahl EC, Tsuchida CA, Gildea HK, Murdock AG, Hochstrasser ML, O’Brien E, Ciling A, Tsitsiklis A, Worden K, Dugast-Darzacq C, Hays SG, Barber CC, McGarrigle R, Lam EK, Ensminger DC, Bardet L, Sherry C, Harte A, Nicolette G, Giannikopoulos P, Hockemeyer D, Petersen M, Urnov FD, Ringeisen BR, Boots M, and Doudna JA on behalf of the IGI SARS-CoV-2 Testing Consortium. PLoS One
