Discovery and development of a CRISPR-based therapy for sickle cell disease
Project Overview
We are collaborating with clinicians at UCSF Benioff Children’s Hospital and the UCLA Broad Stem Cell Research Center to develop CRISPR-based genome editing approaches for real world therapies to treat sickle cell disease.
Genetic diseases of blood cells are prime candidates for treatment through ex vivo genome editing of hematopoietic stem/progenitor cells (HSPCs). We are working to better understand and develop therapeutic approaches for treating sickle cell disease. Sickle cell disease (SCD) is a recessive genetic disorder caused by a single mutation in the β-globin gene (HBB). Sickle hemoglobin damages red blood cells and distorts them into a “sickle” shape, which leads to painful circulatory blockages, progressive organ damage, and premature death.
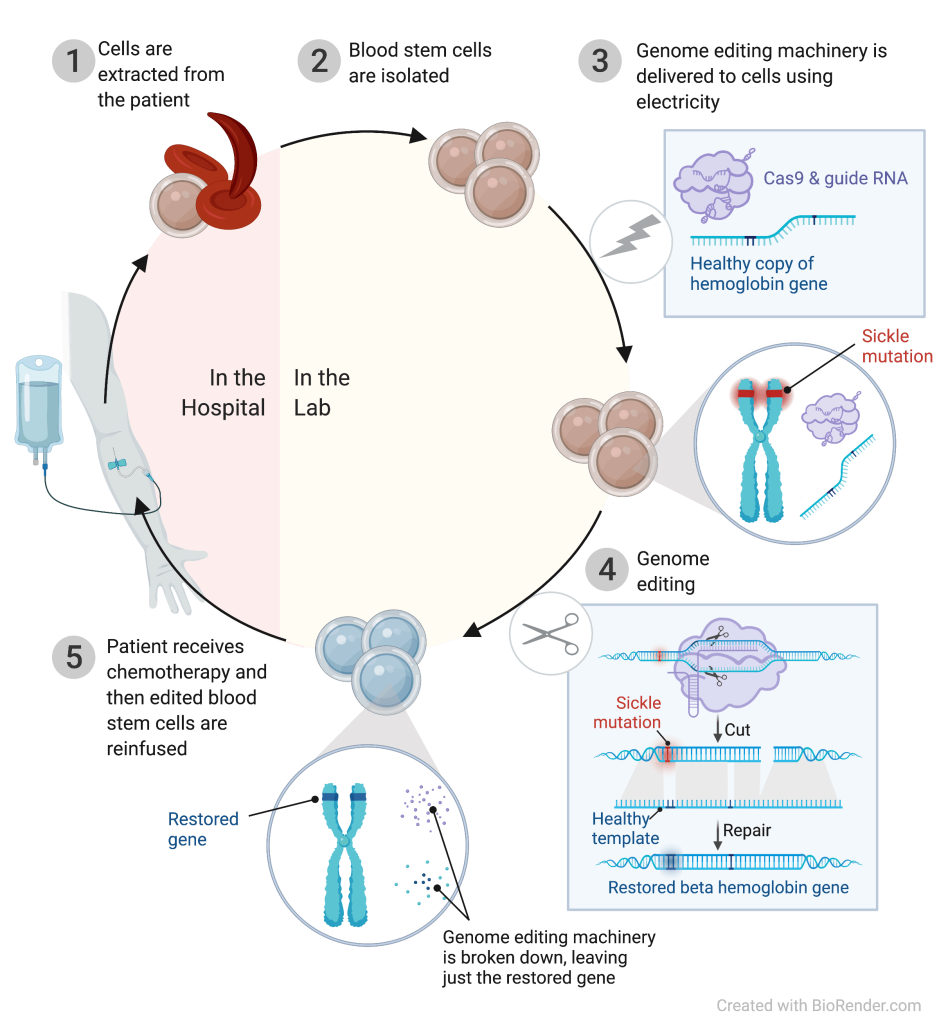
We are developing genome editing into a viable therapy for sickle cell patients. We employ a ribonucleoprotein (RNP) complex consisting of Cas9 protein and single guide RNA (sgRNA), together with a single-stranded DNA oligonucleotide donor (ssODN), to enable efficient replacement of the SCD mutation in human HSPCs. We have already achieved successful editing in patient HSPCs, demonstrated safety and efficacy in a mouse model, and are now working towards a human clinical trial.
Read more:
- IGI and UC Consortium Launch Clinical Trial on CRISPR Therapy for Sickle Cell Disease (2021)
- Making Genetic Therapies Affordable and Accessible: IGI’s New Recommendations(2023)
- IGI Receives Two NIH Awards to Accelerate CRISPR Therapies (2023)
- Revolutionary Care: An Oakland Story (2022)
- $17 Million Will Launch Trial of CRISPR Cure for Sickle Cell (2021)
- The Path to Curing Sickle Cell Disease (2021)
Principal Investigators
- Fyodor Urnov
- Donald Kohn
- David Martin
- Mark Walters
Researchers
- Stacia Wyman
Project Manager
- Zule Romero Garcia
Former Researchers/Collaborators:
- Ron Baik
- Mandy Boontanrart
- Jacob Corn
- Dana Carroll
- Mark DeWitt
- Jenny Shin
- Jonathan Vu
Publications
High-level correction of the sickle mutation is amplified in vivo during erythroid differentiation
Magis W, DeWitt MA, Wyman SK, Vu JT, Heo S-J, Shao SJ, Hennig F, Romero ZG, Campo-Fernandez B, Said S, McNeill MS, Rettig GR, Sun Y, Wang Y, Behlke MA, Kohn DB, Boffelli D, Walters MC, Corn JE, and Martin DIK. iScience
Identification of novel HPFH-like mutations by CRISPR base editing that elevate the expression of fetal hemoglobin
Sam Ravi N, Wienert B, Wyman SK…DeWitt MA, Crossley M, Corn JE, and Mohankumar KM. eLife
Genome editing to model and reverse a prevalent mutation associated with myeloproliferative neoplasms.
Baik R, Wyman SK, Kabir S, and Corn JE. PLoS One
ATF4 regulates MYB to increase γ-globin in response to loss of β-globin.
Boontanrart MY, Schröder MS, Stehli GM, Banović M, Wyman SK, Lew RJ, Bordi M, Gowen BG, DeWitt MA, and Corn JE. Cell
CRISPR-Cas9 interrogation of a putative fetal globin repressor in human erythroid cells.
Chung JE, Magis W, Vu J, Heo SJ, Wartiovaara K, Walters MC, Kurita R, Nakamura Y, Boffelli D, Martin DIK, Corn JE, and DeWitt MA. PLoS One
Improving gene editing outcomes in human hematopoietic stem and progenitor cells by temporal control of DNA repair.
Lomova A, Clark DN, Campo-Fernandez B, Flores-Bjurström C, Kaufman ML, Fitz-Gibbon S, Wang X, Miyahira EY, Brown D, DeWitt MA, Corn JE, Hollis RP, Romero Z, and Kohn DB. Stem Cells
Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells.
DeWitt MA, Magis W, Bray NL, Wang T, Berman JR, Urbinati F, Heo S, Mitros T, Muñoz DP, Boffelli D, Kohn DB, Walters MC, Carroll D, Martin DK, and Corn JE. Science Translational Medicine. Preprint (free to read).