When Will CRISPR Cures Be Available?
Victoria Gray was born with a genetic mutation that causes sickle cell disease. Just one DNA letter change in her genetic code created a faulty protein, leading to years of fatigue, episodes of sudden, intense pain, and long, frequent trips to the hospital. In 2019, Gray became the first person with sickle cell disease to be treated in a clinical trial using CRISPR to restore the faulty protein to its healthy form. Since then, her symptoms have decreased dramatically – no more hospitalizations, blood transfusions, or missing out on special moments with her family. “This is really a life-changer for me. It’s magnificent, ” Gray told NPR.
Gray is not alone. Over 200 million people worldwide are living with rare and neglected diseases. Many of these diseases are caused by small changes in DNA sequences, often just a change in a single DNA letter.“The miraculous success of Gray and other patients in Vertex’s CRISPR trial for sickle cell disease shows the life changing potential of CRISPR cures,” says Fyodor Urnov, Director of the Center for Translational Genomics at the IGI, which focuses on accelerating the development process for new CRISPR-based therapeutics.
At the IGI, we often get messages from individuals with rare and neglected diseases, and their loved ones, asking, “When will we get CRISPR cures?” What will it take for the potential of CRISPR to turn into real therapies that are widely available?
The path to CRISPR cures for rare and neglected diseases
The potential for CRISPR cures creates hope, but also brings up challenges for scientists, drug makers, and regulators. How do you test a treatment if you don’t have enough patients for a clinical trial? How do you decide if a treatment is safe enough if only a handful of people – or just one person – has been diagnosed with the disease it could treat? How do you drive research forward when there are too few patients for pharmaceutical and biotech companies to make a profit?
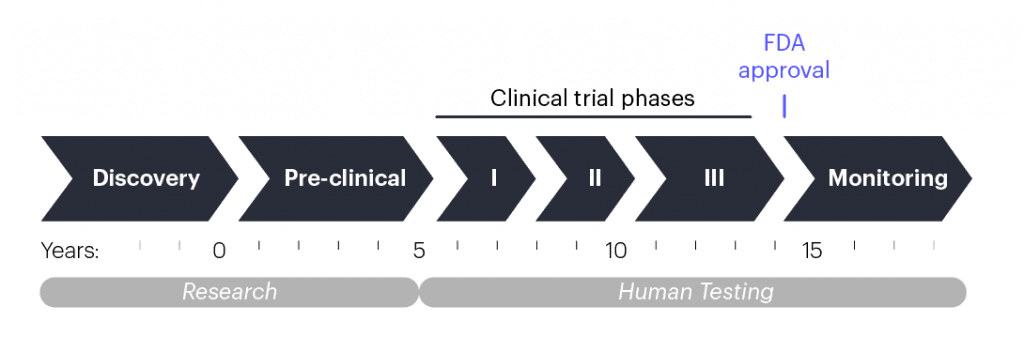
How do treatments usually get to the doctor’s office?
When scientists have an idea for a new treatment, they begin pre-clinical research, which involves lab experiments to make the right formulation and test the treatment in isolated human cells and lab animals. This process takes multiple years and, in the United States, it concludes when researchers submit their data and a plan for a human test called a clinical trial to the U.S. Food and Drug Administration (FDA). If the data and plans are sound, the FDA will let the researchers start testing if the treatment is safe and effective. Other regions of the world have their own versions of the process that follow similar steps.
Clinical trials are done in phases that each take multiple years. Phase 1 clinical trials usually include just a handful of people, and the goal is to make sure that the experimental treatment is safe. As phases progress, the number of patients enrolled increases, and in addition to safety, clinicians start assessing whether the treatment improves patient health. After the third or fourth phase, the FDA or similar governing bodies in other countries may approve the treatment for broad public use if the data show that it is safe and effective. From pre-clinical research to approval, developing a new therapy usually takes 10–15 years.
Rare and neglected diseases face additional hurdles. Financial incentives are the largest problem: developing a new disease treatment is incredibly expensive. Pharmaceutical and biotech companies take on these expenses based on predicted profit from a successful treatment, and the larger the patient group, the larger the potential profit. The smaller the patient group, the less chance to recoup research and development costs and make a profit.
“The fact that genome editing makes it possible to address all genetic diseases in principle doesn’t mean that biotech companies will take on every disease in practice. In the best case scenario, it takes three years to get to a clinical trial, which can cost more than $6 million per disease. Those are big hurdles for realizing the promise of CRISPR and for addressing unmet medical needs,” says Urnov.
The FDA developed the Orphan Drug Designation Program to create special financial incentives for developing treatments for rare (or “orphan”) diseases. This program has helped lead to the creation of 400 new therapies since its inception in 1983. However, 95% of the 5000 rare genetic diseases we know about still have no disease-modifying treatments. We need new approaches to promote the development of products for rare or personalized genetic therapies.
What’s different about gene-editing therapies?
Gene-editing therapies pose some unique technical challenges for both researchers and regulators.
- Delivery: Many researchers in the field consider delivery — figuring out how to get gene editing therapies to the right cells, and only those cells — the biggest technical challenge for gene-editing treatments. The IGI’s Delivery Collective was assembled to tackle this.
- Unknown side effects: There’s extra caution from researchers and regulators because the area is so new and the potential side effects of gene-editing treatments are still being uncovered. Researchers are focused on ensuring that gene editing is only occurring in the targeted areas of the genome, and that there are no potentially harmful immune system responses to the treatments.
- Lack of companion diagnostics and other assessment measures: Companion diagnostics are tests that measure the effect of a treatment. For a gene-editing treatment, researchers might want to know what percentage of cells are edited or if there’s a change in the amount of a disease-causing protein. For rare diseases, these kinds of assessments usually don’t exist, and the cost of creating them is an obstacle. IGI’s Clinical Lab is currently developing companion diagnostics for the UC consortium CRISPR trial for sickle cell disease.
Platform technologies: a way forward for CRISPR cures
If you look at the numbers — 5000 diseases, 10–15 years for each therapy — the timeline seems impossibly long, even with institutions around the world tackling different diseases simultaneously. What can be done to accelerate the process?
One solution for rare genetic diseases could be a platform technology approach. A platform technology is when all the parts of a treatment are standardized into a single off-the-shelf package and only certain pieces of the platform change for a given disease. You can think of it like ordering a burrito: every burrito at your local shop might have the same tortilla, beans, rice, guacamole, and sour cream – this is the platform. The only thing that changes is the protein component that meets the needs of the person ordering the burrito: carnitas, grilled chicken, tofu al pastor, etc.
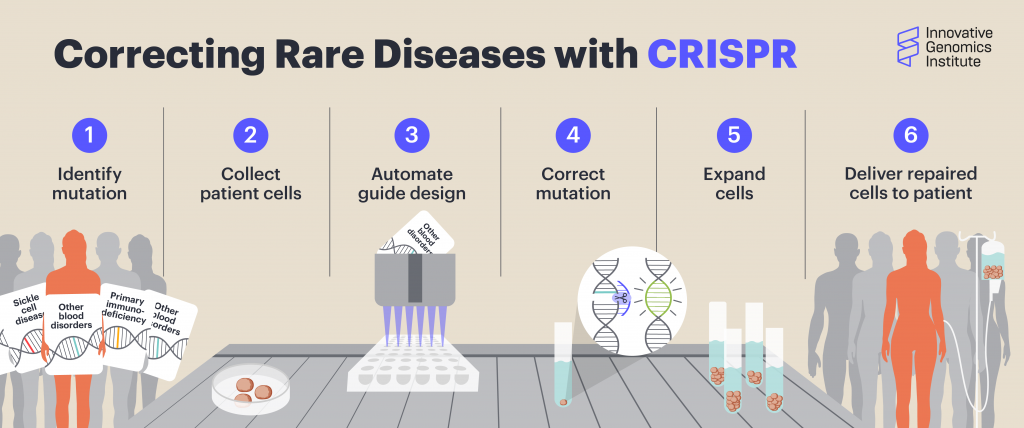
In CRISPR genome editing, a guide RNA molecule brings a Cas protein to a specific spot in the DNA, determined by the sequence of the guide. The Cas protein makes a cut in the DNA at that spot, and then DNA letters can be added, removed, or changed using a custom DNA template. So for CRISPR-based treatments, the basic burrito would be the Cas protein that cuts DNA, the method of delivery to the cells, how the treatment is administered, and what dose a patient gets. The only thing that would change would be the sequence of the guide RNA and any DNA repair templates that need to be specified for different edits.
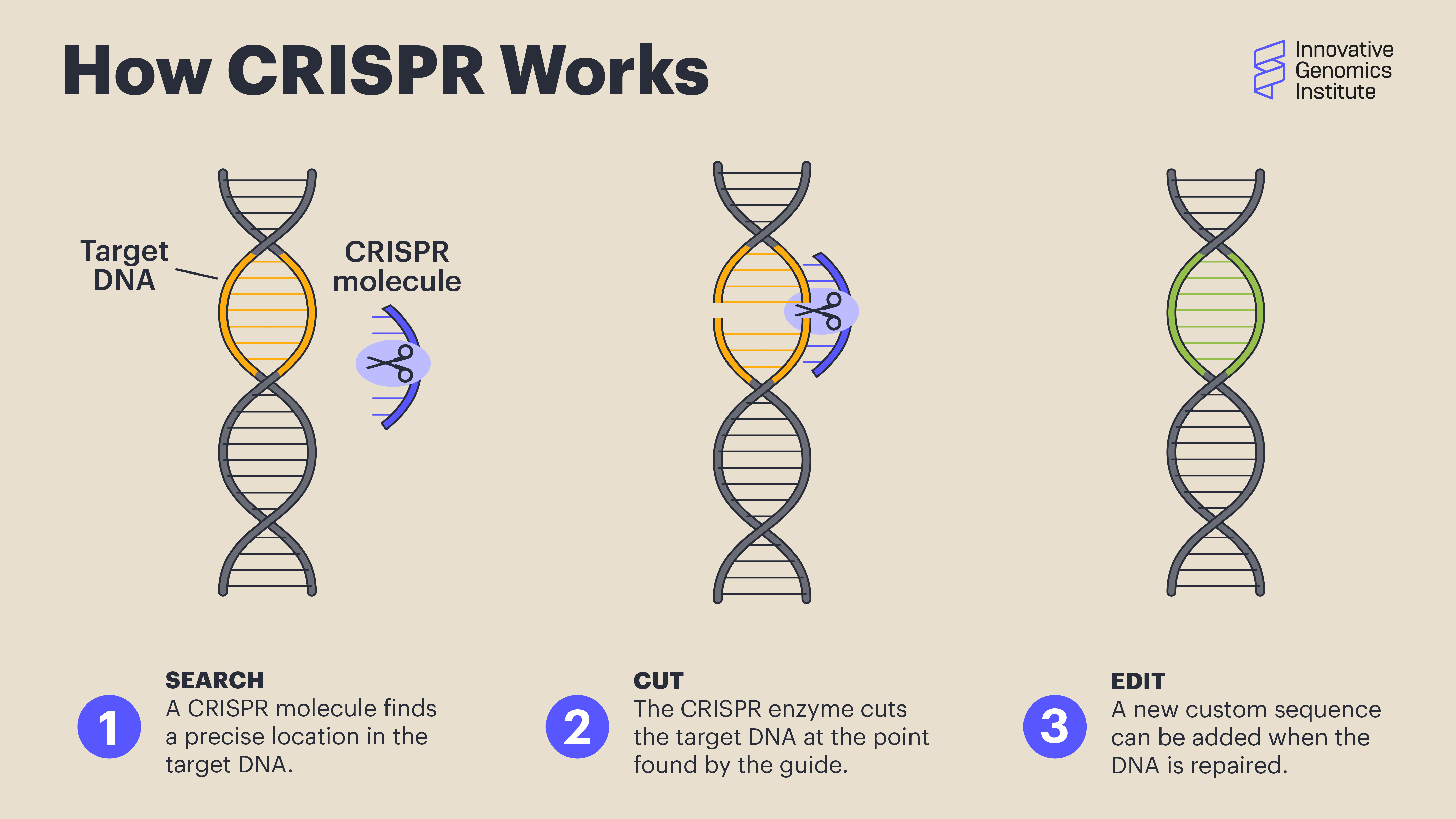
Scientists use CRISPR to find a precise location in the target DNA using a custom-made guide. A CRISPR-associated enzyme makes a precise cut in the target DNA. The cell repairs the break in its genome with the help of a new piece of DNA designed by the researcher.
Ultimately, the idea is that once the various parts of a platform are approved for one treatment, they wouldn’t need to be individually tested and approved again and again, dramatically cutting down on research, development, and clinical testing time, as well as on cost. For more common neglected diseases, this would dramatically lower the time and cost of the research needed to get a clinical trial started. For ultra-rare diseases, the FDA could use Emergency Use Authorizations to grant permission to use the platform technology to treat small groups of patients, or even a single patient. Indeed, the FDA is actively working to develop guidance for new genetic technologies that could benefit patients with ultra-rare diseases.
Developing platforms at the IGI
Creating new platform approaches for curing genetic diseases is the focus of the IGI Center for Translational Genomics (CTG), housed on the first level of the IGI Building in Berkeley. The current flagship project of the CTG is refining approaches for editing blood stem cells.
“Work on editing blood stem cells started with the goal of creating a new therapy for sickle cell disease, but editing these cells also has the potential to cure primary immunodeficiency disorders that leave people severely immunocompromised, and eventually, blood cancers like leukemia,” says Urnov.
A CTG team led by Linda T. Vo is currently developing a high-throughput, automated system to design and test thousands of guide RNAs for the ~500 genes known to cause bone marrow failure syndromes and primary immunodeficiencies, which together affect ~800,000 people in the US alone. With this pipeline, they are building a library of guide RNAs, ready to be used to target these mutations in patient cells.
“Treating sickle cell disease and primary immune disorders might seem like a drop in the bucket in the sense that there are 5,000 genetic diseases. But the centerpiece to our vision is that specific innovations we put together form part of a platform solution, where going from treating one condition to another will be much faster, cheaper, and more scalable than going back to square one,” says Urnov. “We are really hopeful to realize this in partnership with UCSF clinical teams over the next five years. At that point, I can see it taking less than a year to identify a genetic disorder of the blood to having a CRISPR treatment in the works. IGI and UCSF would be able to treat only a small fraction of people in the United States, much less the world, but because we’re a public university and a nonprofit, we will make widely available the blueprint and recipes in the hopes that the IGI approach to CRISPR cures on demand would be replicated more broadly worldwide.”
 By
Hope Henderson
By
Hope Henderson