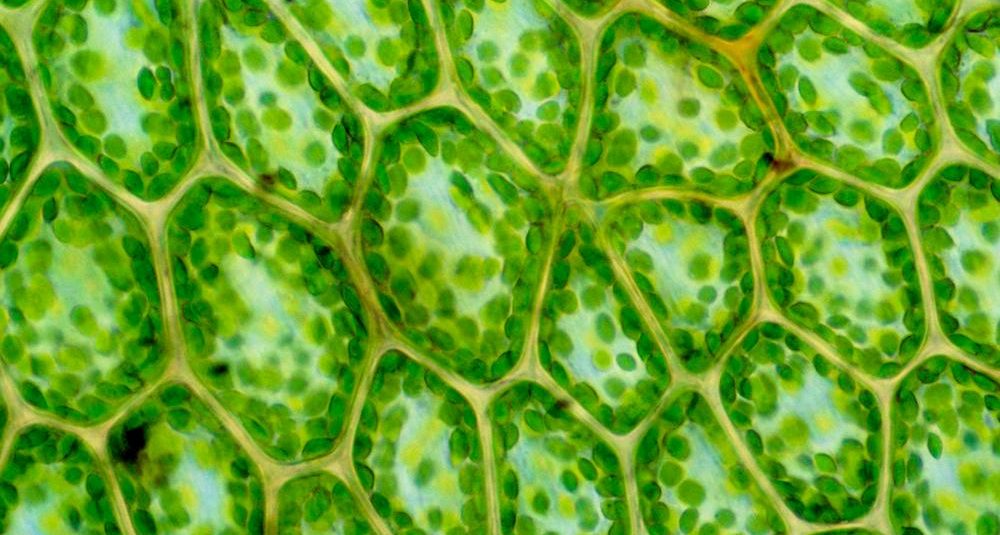
Can Photosynthesis Be Engineered to Be Faster and More Efficient?
New IGI research suggests that there are ways to make the world’s most abundant enzyme better at its job — and better at capturing atmospheric carbon.
Researchers around the world are working on new technologies to capture carbon from the atmosphere, but many approaches fall short on one key metric: their ability to scale. Nature, however, has already developed its own solution to this problem: plants, algae, and photosynthetic bacteria are the world’s best tools for removing carbon dioxide (CO2) from the atmosphere. The majority of this work is done by one enzyme: Rubisco, the most abundant enzyme on earth, responsible for capturing ~100 gigatonnes of carbon every year.
Rubisco is arguably the single most important enzyme on the planet, but it’s far from perfect: compared to many other enzymes it’s quite slow, and it can make mistakes, accidentally reacting with oxygen instead of CO2. In natural systems there appears to be a tradeoff: variations of Rubisco that make fewer mistakes are slower, and faster versions are more error-prone. In a new paper in Nature published today, a team led by IGI Investigator Dave Savage and first author Noam Prywes mapped a large landscape of mutant Rubisco molecules far beyond what has been observed in nature, and found new, unexplored ways to improve and customize its function.
“Engineering Rubisco would be incredibly impactful, as we could improve plants’ ability to assimilate CO2 and, in particular, for adapting to future atmospheric conditions,” says Savage.

Exploring the Rubisco landscape
While there are variations of the Rubisco molecule across the branches of the tree of life, optimized for different environments, millions of years of evolutionary tinkering hasn’t resulted in a version of the enzyme that is both fast and accurate, at least not one that scientists have found. But even if a tradeoff between speed and accuracy is inherent to the enzyme — an open question — genetic engineering could allow scientists to pick versions with the best balance for their specific applications in crop engineering, biological carbon capture, bioengineering, and more.
“What we wanted to do is create high-throughput data sets of Rubisco function so that we could understand its trade-offs better,” says Prywes. “If you can create data sets of Rubisco function, you could also in principle select for Rubiscos that have behaviors that you want for a specific application.”

In this project, the team was looking for how different mutations affect the speed of the enzyme, and its affinity for CO2, which determines its accuracy. To do this, the team worked with their collaborators in the lab of Ron Milo at the Weizmann Institute to engineer a strain of the bacterium E. coli to be dependent on Rubisco. In nature, E. coli doesn’t use Rubisco at all, but the engineered strain can’t survive without it. The team was able to correlate the growth of the bacteria with the speed of its Rubisco molecule — faster growth, faster enzyme — giving them a method of measuring the impact of changes to the DNA sequence that encodes the molecule.
“We did what’s called a ‘deep mutational scan’ where you make every single amino acid mutant of the enzyme. It came out to a little under 9,000 mutants. Then we screened all of them in a pool,” says Prywes.
The question was whether any of these single point mutations meaningfully changed the performance of the Rubisco molecule in the presence of CO2. Most mutations had no effect, while some reduced its affinity for CO2, making it more error-prone. But a few stood out as very different from the rest. “The fun surprise for us was that there were four mutations that were outside of all the noise that improved the affinity for CO2,” says Prywes.
The team experimentally validated two of these mutations, predicting that they would show modest accuracy improvements of around 20% or less, but they were in for another fun surprise. One mutant had roughly doubled the CO2 affinity, and the other roughly tripled it. As in natural systems, they did not escape the tradeoff: while these mutants had dramatically improved their CO2 affinity, they were very slow workers.
“We were excited that such large changes were possible with just a single mutation but it’s important to remember this is just the beginning,” says Savage. “By combining more data and machine learning approaches, we think we can engineer improved variants for use in plants and, possibly, move beyond the tradeoff.”
The team is now repeating the experiment from the opposite direction: testing mutations with oxygen instead of CO2 to look at error rates. The ultimate goal is to fill in the full map of the Rubisco landscape so that it can be custom-engineered for specific applications.
This research was funded in part by the National Institutes of Health grant K99GM141455-01 (NP), the U. S. Department of Energy, Physical Biosciences Program, Award Number DE-SC0016240 (DFS). Dave Savage is an Investigator of the Howard Hughes Medical Institute.
Read more:
- A map of the rubisco biochemical landscape. Prywes et al. (2025), Nature. https://www.nature.com/articles/s41586-024-08455-0
 By
Andy Murdock
By
Andy Murdock