The Path to Curing Sickle Cell Disease
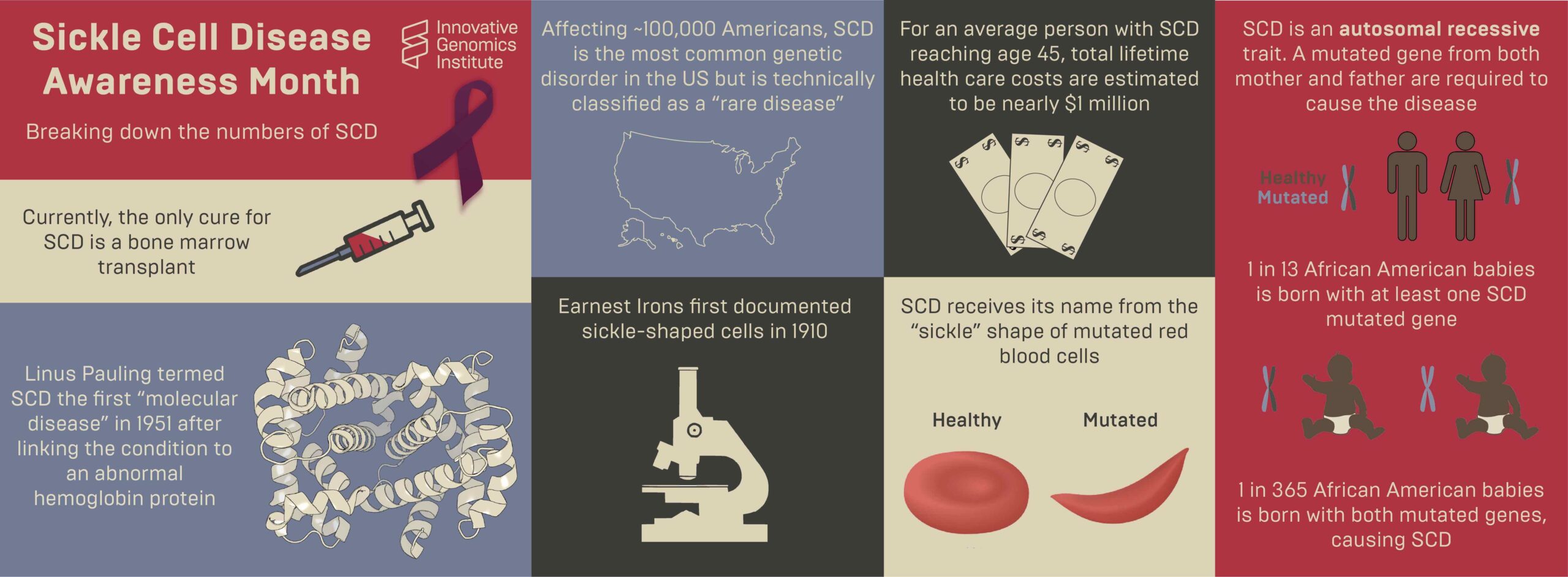
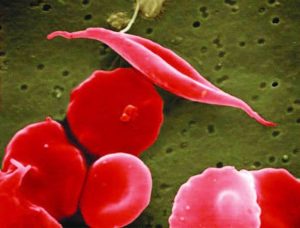
Sickle cell disease (SCD) is a genetic blood disease that affects ~100,000 Americans, and millions more worldwide. It is caused by a single nucleotide mutation in the adult beta-globin gene. Beta-globin protein is a building block of hemoglobin, which carries the iron and oxygen that give red blood cells their color. Beta-globin with the sickle cell disease mutation has a flaw: it causes hemoglobin to clump up within the red blood cells. These clumps distort and weaken the cell, which adopts an elongated “sickle” shape. Sickled cells have a hard time fitting through our smallest blood vessels. They clump up and block the blood vessels, starving nearby cells of oxygen. This progressively wreaks havoc on the body. Severe pain episodes, called “pain crises,” are an early symptom. Organs become progressively and irreversibly damaged. Ultimately, life-threatening symptoms appear: stroke, pulmonary hypertension, and acute chest syndrome. Even in the United States, the life expectancy of individuals with sickle cell disease is only 45 years. As Shakir Cannon poignantly illustrated in his post “Prioritizing Sickle Cell Patients: A Chance to Mend Broken Ties,” quality of life with sickle cell disease is dramatically affected as well.
The standard of care for individuals with more advanced sickle cell disease is chronic blood transfusions coupled with chelation therapy, which has severe side effects. Blood cells are made in the bone marrow, and for some patients, a bone marrow transplant (BMT) is an option that can cure sickle cell disease. In a BMT, a patient undergoes a course of chemotherapy to wipe out their bone marrow cells. Then, they get an injection of healthy cells from a donor. These cells take up residence in the bone marrow, ultimately producing healthy red blood cells. BMTs are risky and intensive, and they are limited by cost and availability of a matching bone marrow donor. That’s the bad news. The good news is that new technologies may dramatically improve the outlook for individuals affected by SCD in the coming years.
The brightest future for sickle cell disease lies in so-called “autologous” procedures. These are procedures that genetically correct a patient’s own (“auto” means “self”) bone marrow cells in a lab. After chemotherapy, the corrected cells can be injected back into the patient, where they engraft and produce healthy red cells and cure the disease.
Autologous curative therapies for sickle cell disease are taking two routes: gene therapy and gene editing. In gene therapy, a harmless virus is used to add a copy of a healthy beta-globin gene to cells. If enough cells get this new gene, they will express enough healthy beta-globin, fending off sickling. This approach is currently in clinical trials run by several academic and private sector organizations.

The other approach is genome editing. The first genome editing trial for sickle cell disease is using CRISPR to turn on fetal hemoglobin, a form of hemoglobin that fetuses have but stop making shortly after birth. In this approach, fetal hemoglobin does the job of beta-globin. The first patient, Victoria Gray, was treated in 2019. Gray and other trial participants have made remarkable progress. You can learn more about this approach and clinical trial results here, and hear from Gray herself here.
Managing sickle cell disease is a complex challenge for doctors and patients, but the genetic cause is very simple: one single letter mutation in one single gene. Instead of addressing the problem by turning on fetal hemoglobin, it may be possible to directly fix the disease-causing mutation. We can do this by using CRISPR genome editing components to cut DNA at the site of the mutation, and add a healthy DNA sequence that the cell can use to repair the DNA. When the DNA repair process is complete, the disease-causing mutation has been replaced with healthy DNA sequence.
The IGI together with a consortium of University of California researchers at UC Berkeley, UCSF, UCLA, has developed a treatment to directly fix the disease-causing mutation with CRISPR technology. Clinical trials being held in Oakland and Los Angeles will begin enrolling patient volunteers in the summer of 2021. Learn more about the trial here and here.
Right now, gene therapy and gene editing treatments for sickle cell disease both require a bone marrow transplant, the cost is prohibitively high, and the therapy is not accessible around the world in the places where it can do the most good. Autologous transplants are a big leap forward in treatment, but IGI researchers are already working on version 2: eliminating the need for chemotherapy. While the next generations of CRISPR-based sickle cell therapies are some years down the road, the IGI is actively working toward a goal of being able to safely edit bone marrow stem cells with a simple injection, and have this therapy be affordable and accessible to all who need it.
You may also be interested in

Prioritizing Sickle Cell Patients: A Chance to Mend Broken Ties
Vence Bonham Seminar: Searching for a Cure – Sickle Cell Disease Gene Editing

IGI Seminar Series: Progress and Challenges in Delivering Cassava with RNAi-mediated Resistance to Cassava Brown Streak Disease to Smallholder Farmers in Africa – It’s Not Just About the Technology