IGI Receives Two NIH Awards to Accelerate CRISPR Therapies
Research teams are developing new therapies for neurodegenerative diseases and clinical tests to aid FDA approval
Just over a decade since the development of CRISPR genome editing by Innovative Genomics Institute founder Jennifer Doudna and collaborator Emmanuelle Charpentier, the National Institutes of Health (NIH) is using its funding might to incentivize researchers to think beyond single applications of genome editing and to develop platform approaches that can be used to address multiple diseases. In two awards totaling $25 million just announced from the NIH’s Somatic Cell Genome Editing (SCGE) program, researchers at the IGI are developing new approaches to treat multiple neurodegenerative diseases as well as clinical tests to help new CRISPR-based therapies reach patients.
The first project, dubbed “Correction of Neurological Disease via Allele Specific Excision of Pathogenic Repeats” or “CASEPR” (pronounced “case-per”), brings together a group of experts in CRISPR genome editing, neurological medicine, and regulatory requirements, with the goal of reaching a clinical trial in five years. The team includes Jennifer Doudna, Fyodor Urnov, Niren Murthy, Petros Giannikopoulos, and Ross Wilson at the IGI at UC Berkeley, Krystof Bankiewicz and Victor Van Laar from Ohio State University, and Claire Clelland at UC San Francisco.

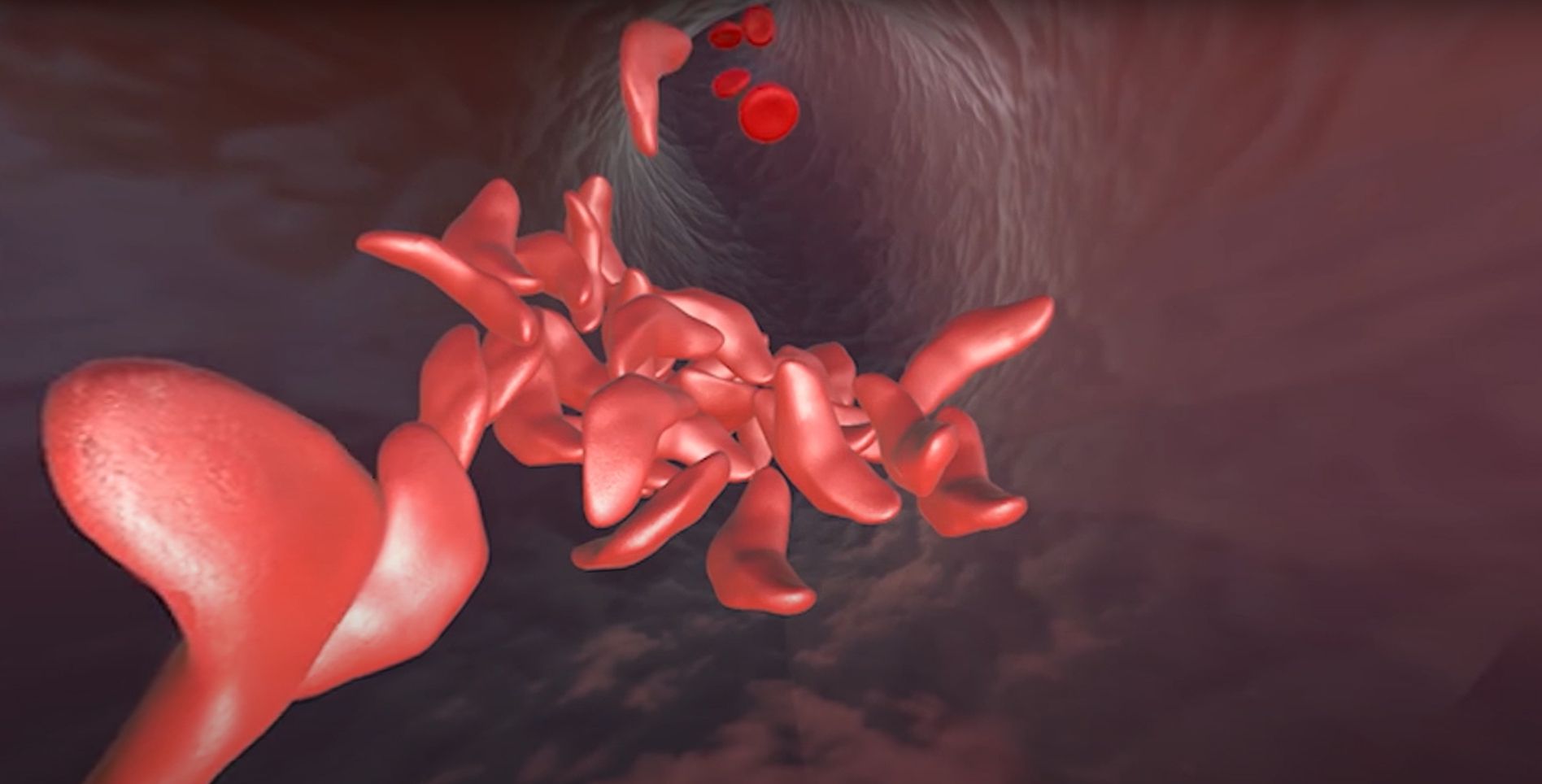
While some genetic diseases are caused by single point mutations where one letter of the genetic code is changed, several neurodegenerative diseases are caused by a different type of mutation called an “expansion repeat” where short strings of DNA code get accidentally duplicated when DNA is copied or repaired by our cells. These small duplications can transform a healthy copy of a gene into a pathogenic one, particularly if this error happens multiple times.
“In Huntington’s disease, it’s a very clear correlation. There’s a gene containing a variable number of copies of the sequence ‘CAG’ — CAG, CAG, CAG, over and over — if you have too many copies, you get sick. The more copies you have, the sicker you are, the more rapid the progression,” says Ross Wilson, Director of Therapeutic Delivery at the IGI.
The challenge in designing a genomic therapy is that there are both healthy and pathogenic copies of the gene in a patient, so a genome-editing intervention has to be able to precisely target the gene with too many repeat copies while leaving the healthy gene untouched. In the CASEPR project, the team will be employing a CRISPR technique that can be delivered in vivo and will selectively disable the pathogenic copies. The team will focus on applying this technique to Huntington’s disease and a common form of amyotrophic lateral sclerosis (ALS), sometimes called Lou Gehrig’s disease, with the goal of reaching a clinical trial in five years.
The team will be comparing two different methods of delivering the CRISPR therapies to affected cells using viral vectors that are commonly used in gene therapies, and a non-viral approach developed at the IGI with the aim of creating a road map for a safe, reproducible approach that can be applied to other neurodegenerative diseases in the future.
New Therapies, New Tests
As more and more CRISPR-based therapies march toward the clinic, researchers — and regulators — need reliable ways to assess the safety and efficacy of these therapies. But because genome editing is still so new, clinical-grade tests have to catch up with the field.

On a second project that received a 3-year award through the NIH’s SCGE program, Petros Giannikopoulos, Director of the IGI Clinical Laboratory, is leading a group developing a clinical-grade test that can be applied across the multiple ongoing trials using genome editing to treat sickle cell disease. Some groups are taking the approach of using CRISPR to reactivate a form of hemoglobin that is not affected by the sickling mutation, while others, including the UC consortium trial that the IGI is involved with, are directly correcting the underlying mutation. While the current generation of therapies happens ex vivo, or outside the body, the goal for the next-generation therapies is to be delivered in vivo, to make the therapies more accessible and easier for patients.
“One of the big challenges in the field is the lack of a consensus method for assessing the functional benefit of a sickle cell treatment,” says Giannikopoulos.
Currently, researchers test for the effectiveness of genome editing by sequencing the DNA after treatment to measure what fraction of the DNA has been corrected, but that isn’t a direct functional measurement of the disease and its effects, which is key for groups seeking FDA approval.
“Sickle cell disease causes trouble in a lot of different ways. There’s a constellation of pathologic properties that a red blood cell acquires. When you correct a stem cell, what you’re trying to look for is a loss of those properties,” says Giannikopoulos.
For the new project, the team at the IGI — working in collaboration with Umut Gurkan at Case Western Reserve University, Mark Walters and Frans Kuypers at UCSF Benioff Children’s Hospital, and UC Berkeley computational biologist Nila Ioannidis — is developing a new composite test using a panel of assays to create a test that applies no matter how genome editing was performed. These include cutting-edge microfluidic assays that can test for properties like stickiness and flexibility of red blood cells, as well as liquid chromatography tests that have long been used to diagnose sickle cell disease.
“There isn’t one killer app — we’re trying to take a comprehensive approach. We feel really good that this is doable, and we have all of the technical and clinical expertise to get it done,” says Giannikopoulos. “For anyone who’s working on sickle cell therapies, this is a key part of getting those therapies across the regulatory finish line.”
Award Details:
- NIH Award 1U19NS132303-01 — Correction of Neurological Disease via Allele Specific Excision of Pathogenic Repeats
- NIH Award 1U01AI176469-01 — A Modality-Agnostic Potency Assay Enabling Both Ex Vivo and In Vivo Genome Editing Therapeutics for Sickle Cell Disease
Media Contacts:
- Andy Murdock, Innovative Genomics Institute — andymurdock@berkeley.edu
 By
Andy Murdock
By
Andy Murdock