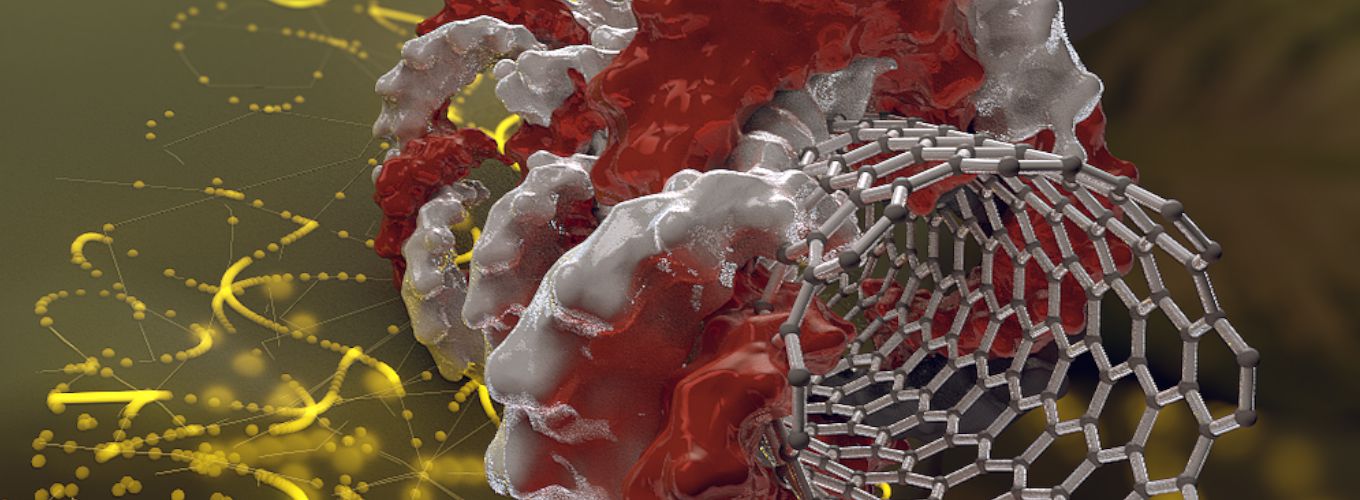
DNA Takes a Ride on a Nanotube: Next Stop, Genome-Edited Crops
IGI scientists use nanotechnology to sneak DNA into plant cells, creating a novel delivery method for CRISPR-Cas9.
How do we feed a growing population as the world heats up? Genome editing is key to making crops that can withstand weather and ecosystem changes brought on by climate changes, but delivering genome editing tools to plants is a major challenge. In a paper published today in Nature Nanotechnology, IGI researchers at UC Berkeley report a major advancement in plant genome editing: using carbon nanotubes to deliver DNA to plant cells for genome editing.
Tools for editing plants
Plants pose a tough challenge to would-be genome editors. Like animal cells, plant cells are edited using tools like DNA, RNA, and proteins that need to get past the cell’s membrane, and then find their way into the cell’s nucleus, the compartment where the genome is housed. Unlike animal cells, plant cells have a cell wall: a protective outer layer made of tough plant fiber. This rigid structure helps give plants cells their shape, and protects them from infection and dehydration—but also acts as a fence, keeping out big molecules, like genome-editing proteins.
Currently, scientists use a few methods to get past the cell wall. One common method is co-opting a bacteria that naturally infect plants to deliver DNA with genes for desired traits—in other words, taking advantage of ways microorganisms have already figured out to get past the cell wall. If a bacteria cannot be engineered to infect a certain plant species, the next approach is a “gene gun”: researchers coat metal nanoparticles in DNA and “shoot” them into plant cells like tiny bullets.
These methods share some common drawbacks: both often have low efficiency, meaning the DNA only gets into a small percentage of cells, and most cells will actually not have their genomes edited. Furthermore, the DNA that does get in is incorporated into the plant’s own genome at random locations. Sometimes those locations are inside the DNA sequences of important genes, which stops them from working.
Enter scene: carbon nanotubes
Collaboration across disciplines sparks innovation, and the IGI brings together researchers in different fields to do just that. Two IGI researchers, Markita Landry, a chemist and bioengineer, and Brian Staskawicz, a plant biologist, worked together to find a new, nanotechnology approach to editing plants.
Carbon nanotubes are tiny, hollow cylinders, just one nanometer in diameter, made entirely of carbon. Their unusual molecular properties, like strength, flexibility, and ability to conduct heat, make them valuable for a variety of applications in nanotechnology, electronics, and related fields.
Landry, Staskawicz, and researchers in their labs worked together to coat the surface of carbon nanotubes in DNA. The cell wall acts like a fence, keeping most molecules out. But because carbon nanotubes are so narrow, they can slip through the cell wall, like slipping through the links in chain link fence. In their experiments, the IGI team found carbon nanotube delivery worked with 85–95% efficiency—dramatically higher than current technology—with no harmful side effects.
Delivering CRISPR on carbon nanotubes
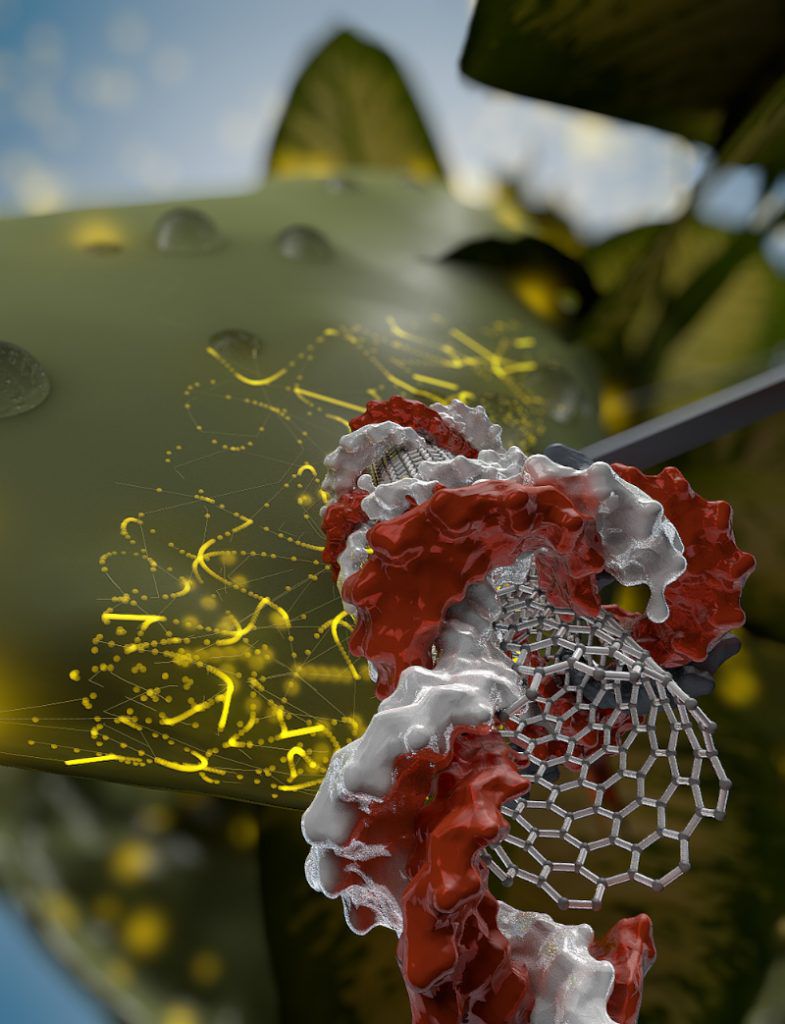
When carbon nanotubes deliver DNA to plant cells, the cells use the DNA as a template to make proteins and RNA. Within about ten days, natural enzymes in the cell break down the nanotube-delivered DNA. The IGI team wants to use this to deliver CRISPR-Cas9 genome editing tools in the future: “If we’re expressing Cas9 and its guide RNA—it can edit the genome and within a few days the DNA disintegrates, the Cas9 disintegrates, but you have the edit,” explains Landry.
In this application, the carbon nanotube would be coated in DNA that codes for the Cas9 protein and the guide RNA, which work together to edit a specific gene. The genome editing tools are present just long enough to edit the plant’s DNA before being broken down by the plant, leaving no footprint. The plant’s DNA would only be changed at the one specific site scientists wish to change. This means that unlike with some current plant genome editing methods, no foreign DNA would be inserted into the plant’s genome. If these plants became part of the food chain, people would not be eating Cas9 or any other molecular biology tools: just rice with a gene changed to make it more nutrient-rich, or strawberries with a gene changed to make them less tasty to slugs.
Harder, better, faster, stronger: editing chloroplasts
Many of a plant’s most important genes are shielded within a structure that has remained tantalizingly out of reach: the chloroplast. Chloroplasts are the cell compartment where plants make energy using sunlight in the process known as photosynthesis. Like mitochondria, chloroplasts evolved from bacteria, and have their own genome that codes for many essential photosynthesis genes. Editing chloroplast genes can increase the amount of energy made by photosynthesis, which in turn can make plants grow bigger, faster, and produce more yield. But editing chloroplast genomes is even more challenging than editing the main genome of a plant cell: genome editing tools need to not only get past the cell wall, but find the chloroplast, and get past the chloroplast membrane. Bacterial plant delivery tools cannot do this, and gene guns have very low success.
“When we do tracking studies to see where the nanoparticles end up,” says Landry, “they go to the nucleus and chloroplasts. Estimating that the nanotubes end up in over 90% of chloroplasts suggests that we may have a high-throughput way of delivering genes to chloroplasts that would far exceed the capabilities of current technology.” Carbon nanotubes could become a prime tool for editing the chloroplast genome.
Next steps
“By 2050, we’re going to have to increase food production by 70%. How do we produce plants in a more durable, sustainable way? That’s the challenge,” explains study author Brian Staskawicz. “And I think genome editing allows us to do that, actually. Will allow us to do that–it’s still currently in its infancy.”
If it works, scientists could use this method to act quickly in response to pathogens, insect pests, and drought that threaten vital food crops like soybeans, and to protect our most beloved crops like cacao and coffee, which are under threat from infectious diseases and rising temperatures.
“The amazing thing about these carbon nanotubes is that they’re able to get past the cell wall and go into the nucleus or into the chloroplasts. It’s a novel advance that’s allowing us to really put in place the tools for genome editing,” says Staskawicz. “The next steps would be, can we deliver ribonucleic proteins or can we deliver mRNA or DNA that would actually encode CRISPR-Cas9?” The answers are within reach.
–
This study was published in the online edition of the journal Nature Nanotechnology as “High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants.” In addition to Landry and Staskawicz, co-authors are Gozde S. Demirer, Huan Zhang, Juliana L. Matos, Natalie S. Goh, Francis J. Cunningham, Younghun Sung, Roger Chang, Abhishek J. Aditham, Linda Chio, and Myeong-Je Cho.
The Innovative Genomics Institute (IGI) is a non-profit, academic partnership between UC Berkeley and UC San Francisco that supports collaborative research projects across the Bay Area. The IGI’s mission is to develop and deploy genome engineering to cure disease, ensure food security, and sustain the environment for current and future generations. As pioneers in genome editing, functional genomics, and other cutting-edge technologies, IGI scientists continuously push the boundaries of science.
Media Contacts
Megan Hochstrasser: megan.hochstrasser@berkeley.edu
Markita Landry: landry@berkeley.edu

 By
Hope Henderson
By
Hope Henderson