
From Battlefields to Cancer Wards: CRISPR to Combat Radiation Sickness
Bay Area researchers harness the power of CRISPR to prevent and treat acute radiation sickness.
A team of American warfighters land on enemy soil, and enter a deserted government research building—a suspected site for weapons development. They wear hazmat suits, and carry Geiger counters to detect radiation. The devices give a few beeps as they enter the building. The warfighters comb room after room, finally coming to a room with a heavy steel door. When they open it, the beeps come faster and faster until there is just a steady, high-pitched noise coming from the counters: they are about to enter a zone with a potentially lethal level of radiation. They continue their work, containing nuclear materials that could be immensely dangerous in the wrong hands. But within a few weeks of completing their mission, the warfighters start getting sick. They suffer from severe fatigue, infections, and hemorrhage.
A woman lies in a hospital bed, pale and weak under her cotton hospital gown. Machines whir around her, continuously measuring her vital signs. She has been undergoing combination chemotherapy and radiation therapy for esophageal cancer. Her cancer is shrinking, but the same radiation that kills the fast-growing cells of her tumors also damages nearby cells in the rest of her esophagus and stomach. She is suffering from severe diarrhea, electrolyte imbalance, and dehydration. The only option is to stop radiation therapy—which gives the cancer a chance to bounce back, making a cure less likely.
Taking a Moonshot
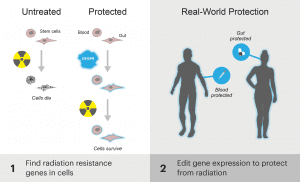
Both of these scenarios describe an area of major unmet medical need: acute radiation sickness, or ARS. Today, the IGI announces funding for a massively ambitious project: using CRISPR-Cas9 to combat ARS. The goal of this project is to find genes that, when turned on or off, protect against ARS, and use CRISPR-Cas9 to temporarily and reversibly change the expression of these genes. Much like getting a vaccine to protect against the flu, scientists aim to produce a single-dose genetic medicine that could be given as a shot or pill that would preemptively protect against radiation. Scientists also aim to develop a post-exposure treatment. Ultimately, the researchers hope to create a genetic medicine that they can take all the way to clinical trials in humans.
UC San Francisco has received a $10 million grant from the Defense Advanced Research Projects Agency, or DARPA, to fund this research in collaboration with UC Berkeley via the IGI. Performance pending, another $10 million will be awarded in two years. The project is led by Jonathan Weissman, IGI Co-Director and Professor at UC San Francisco and Fyodor Urnov, IGI’s Scientific Director for Translation and Technology. The work will be done in collaboration with other researchers at UCSF, UC Berkeley, and Carnegie Mellon University.
“One of the reasons it’s fun to work with DARPA is that they propose the impossible,” says IGI Investigator and UCSF Professor Luke Gilbert, a researcher on the project. It is, “Totally a first of its kind,” says Dr. Mary Feng, Medical Director of the Clinical Research Network Office at the UCSF Diller Comprehensive Cancer Center, who serves as an advisor to the work.
What is ARS?
Currently, ARS is a major unmet medical need in both national defense and cancer treatment. Warfighters, first responders, and civilians are exposed to radiation at the scene of nuclear accidents or attacks. And, about half a million cancer patients in the US alone receive radiation therapy each year. In addition to causing painful, sometimes chronic, symptoms, ARS limits how much radiation doctors can give cancer patients. Patients must take breaks from or stop radiation treatment altogether—which gives cancers a chance to regrow, and reduces the chance of cure.
“In respect to prevention of radiation damage to abdominal organs, there’s really nothing out there,” say Feng. One other drug has been tried, but is is not very effective, and, according to Feng, who ran the clinical trial, “Just impossible to use practically. No one uses it because its side effects are so bad, and the therapeutic index is very low.”
The Path to the Moon
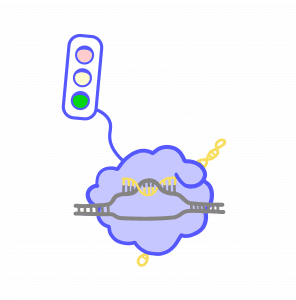
In the CRISPR-Cas9 system, programmable RNAs guide the Cas9 protein to specific locations in the genome. This system has become famous for its ability to edit DNA. Over the last few years, Weissman and Gilbert have pioneered new ways to use CRISPR-Cas9 technology to turn genes on and off without changing the DNA sequence itself. Instead, they use it to change the proteins and molecules that surround DNA. Repressor proteins and molecules work like red lights, telling gene activity to stop. Activator proteins and molecules act like green lights, telling it to go. Using CRISPR-Cas9, scientists can change these proteins and molecules, changing gene expression without making any changes to the DNA code. These changes are temporary and cannot be passed on to future generations.
As a first step towards this goal, scientists will use isolated human cells to screen the entire genome for genes that protect against radiation when they are turned on or off by CRISPR-Cas9. These genes will also be tested in intestinal organoids, which are small collections of intestinal cells that grow together in a dish, mimicking the three-dimensional structure of intestinal tissue better than individual cells can. Next, scientist will investigate the proteins and molecules that modify gene expression, determining which are the best to target.
“A really interesting aspect of this collaboration between the IGI and DARPA is the notion of evoking the body’s own defense mechanisms to try to protect against radiation damage or help recover from it,” says Urnov.
“Some smaller organisms have completely different tolerance thresholds. The cockroach is famously radiation-resistant, as is the much more pleasant tardigrade. What that teaches us is the fundamental concept that Mother Nature is not intrinsically susceptible to the threat of radiation. It is simply a matter of evoking the right defense to the right level.”
Delivery – that is, getting the treatment to the right place at the right time – is one of the biggest challenges of editing the genome, and editing proteins and molecules that turn gene expression on and off. Genome editing often uses modified viruses, taking advantage of the ways viruses have evolved to get into human cells. In addition to testing viral vectors for delivering editors, the researchers will also test a virus-free method using lipid nanoparticles. In this system, editors will be encased in tiny fat bubbles that can safely penetrate into cells.
After exhaustive screening of gene “hits,” optimizing editors, and figuring out the best delivery methods, scientists hope to have a few candidate genetic medicines that turn “hit” genes on and off. They will test them extensively in animal models, looking to protect health before and after radiation exposure. If the medicines are effective in animals, they will move to human clinical trials.
Landing the shot
Ultimately, if the scientists are successful, this work will harness the power of CRISPR-Cas9 to rewrite the ending so the warfighter containing nuclear material takes a pill on their way to the site, and their family rests easy, knowing they are protected. Scientists are working towards a future where the patient with esophageal cancer isn’t poisoned by her treatment, but gets treated with genetic medicine that allows her to receive a curative dose of radiation. “It’s a really futuristic bet,” says Gilbert. “Some part of it will turn into something amazing—hopefully it all does.”

“Biological systems have enormous resilience,” says Weissman. “CRISPR screening technology has revolutionized our ability to explore and manipulate this resilience. We now seek to exploit intrinsic radiation-protective circuits that human cells have.”
“At the University of California we are deeply mindful of the mandate we have to serve the public good, and our efforts with DARPA to use CRISPR as a discovery tool and the therapeutic agent in a radiation countermeasure give us a
wonderful opportunity to put this mission into practice.”
This grant was awarded as part of the Defense Advanced Research Projects Agency’s PReemptive Expression of Protective Alleles and Response Elements (PREPARE) program. The Principal Investigator on this project is UCSF Professor and IGI Co-Director Jonathan Weissman. Other researchers include UCSF Assistant Professor and IGI Investigator Luke Gilbert, who will collaborate with Weissman on the genome-wide screening. UC Berkeley Assistant Professor and IGI Investigator Dirk Hockemeyer will do screening and delivery research on intestinal organoids. Fyodor Urnov, Scientific Director for Technology and Translation at IGI, will work to optimize epigenome editors. Katie Whitehead, Associate Professor at Carnegie Mellon University, will work on non-viral delivery methods. Mary Feng, Medical Director of the Clinical Research Network Office at the UCSF Diller Comprehensive Cancer Center, and Felix Feng, Director of Translation Research at the UCSF Department of Radiation Oncology, will provide input to make radiation studies mimic patient scenarios, and take the lead when this work moves into human clinical trials.
You may also be interested in

Luke Gilbert Named NIH “New Innovator” for Pioneering Potential

Next Generation Guide RNAs for Genome-Wide CRISPR Screens

Alex Marson Appointed IGI Scientific Director of Biomedicine

 By
Hope Henderson
By
Hope Henderson