New Approach to CRISPR Delivery Opens the Door to Editing Multiple Genes Safely
New work from IGI Investigators at UC Berkeley, UC San Francisco (UCSF), and Gladstone Institutes paves the way for the next generation of CRISPR therapies. Published today in Nature Biomedical Engineering, Ross Wilson, IGI Director of Therapeutic Delivery; Justin Eyquem, Assistant Professor of Medicine, UCSF and Affiliate Investigator at Gladstone Institutes; Alex Marson, IGI Director of Human Health and Director at the Gladstone-UCSF Institute of Genomic Immunology; and colleagues demonstrate a new approach to overcome the challenge of efficiently delivering CRISPR genome-editing molecules into living cells: mixing CRISPR components with short snips of proteins called peptides to form a mixture that can slip through the cell’s outer layer.

Genomic medicines and cell therapies like CAR-T cancer immunotherapy, including therapies that involve genome editing, are already impacting patients. But delivery is one of the biggest challenges for creating and improving these treatments. The most common way to deliver CRISPR to isolated cells in a lab uses an electric shock to momentarily disarm a cell, letting the CRISPR components sneak in. But the shock is tough on cells: a significant percentage don’t survive the treatment. In those that do survive, research shows that there are big changes in gene activity.
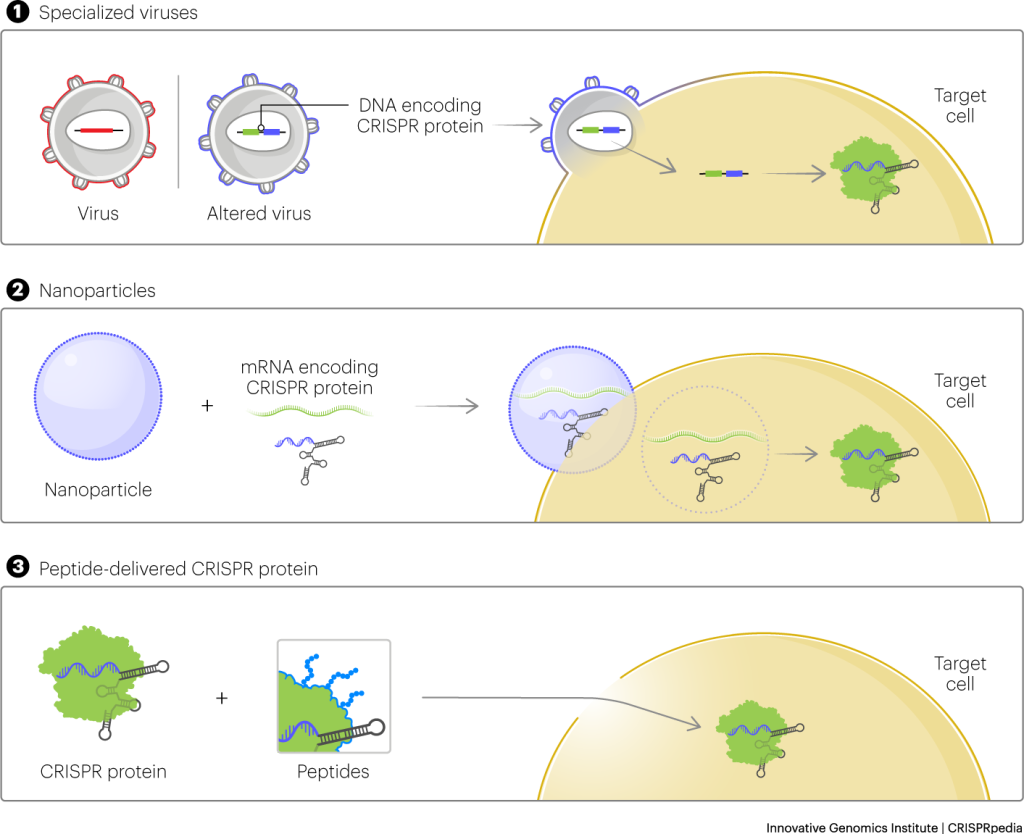
Another conventional delivery approach relies on packaging CRISPR tools inside a virus. The virus can stick around in the body for months or years. The longer the CRISPR components are present, the higher the chance that they’ll make unwanted cuts to the genome.
IGI researchers’ new peptide approach is much gentler on the cell. The peptide is simply mixed with the CRISPR enzyme. The peptides help CRISPR enzymes get into a cell without the need for an electric shock. Few cells die in the process and there is very little effect on overall gene activity. The CRISPR components are present only for about a day, dramatically reducing the chances of unwanted cuts. And with the peptide method, no virus is necessary to edit a gene.
An additional benefit is that the new method will help make it possible for more researchers to work on developing CRISPR-based therapies by making it cheaper and easier. Viruses used for delivery are challenging to make, and the electric shock protocol relies on special equipment.

“Right now there’s a lot of infrastructure that’s required to do these experiments in mammalian cells,” says co-first author David Nguyen, IGI Investigator, Assistant Professor of Medicine at UCSF, and Assistant Professor of Immunology at UC Berkeley. “The peptide method is going to democratize small experiments with gene editing in labs that don’t have the resources to invest in electroporation setups or to make viruses.”
And when those experiments yield new therapies, peptide manufacturing is easy to scale up.
“The earliest progress with CRISPR delivery came from adapting platforms that were already on hand,” says Wilson. “Viruses are an ideal fit for traditional gene therapy where you’re adding an entire new copy of a gene into a cell. Lipid nanoparticles were designed for delivering DNA and RNA. But we want to get CRISPR proteins into the cell, ready to go. Our peptide-driven approach takes advantage of CRISPR itself – we can actually avoid the complexities and limits of these other platforms.”
The most widely used cell therapy product is CAR-T. CAR-T cells are human immune cells that have been engineered to attack cancerous cells in the blood. Usually, they’re made by taking T cells from a patient, in some cases using specialized equipment to give the cell an electric shock, and then using viruses to add older-generation genome engineering tools. What if the multiple genomic changes needed for an effective immunotherapy could all be made with the precision of CRISPR?
It is amazing what is achievable when three labs with diverse expertise pursue a truly collaborative effort to solve a big problem in gene editing.
—Dana Foss
In most conventional CRISPR-based therapies, only a single gene can safely be edited in any given cell. For genetic diseases like sickle cell disease that are caused by a change in a single gene, this can be revolutionary. But for many other diseases – solid tumor cancers, for instance – editing multiple genes (“multiplexing”) in the same cell could be hugely beneficial. Right now, researchers trying to multiplex editing run into a dicey problem. Generally, CRISPR reagents work by making a cut in the DNA. When the cell repairs the cut, it’s an opportunity to change the DNA sequence. But when cuts are made in multiple locations in the genome at once, things can get mixed up.

“If you want to edit multiple genes in the genome, and in doing so make multiple double-strand breaks, this creates the risk of harming the integrity of the genome,” explains Joe Muldoon, co-first author on the new paper and a postdoctoral fellow at UCSF. “The cell will do anything it can to repair DNA breaks. And if that means sticking chromosomes back together in ways that they don’t belong, it’ll do that.”
In recent years, Editas Medicine identified a possible solution to this: instead of doing multiple edits all at once, they could theoretically be done one by one, waiting a couple days between each edit. That way, the cell has time to repair the cuts between each change, limiting the potential for unwanted chromosomal rearrangements. However, at the time Editas identified this approach, there was no real way to do it: applying multiple rounds of electric shock would leave few cells alive at the end.
With the gentler peptide approach described in the new paper, Wilson and collaborators have shown that it’s possible to make up to three edits in a row in the same cells without resulting in a massive die-off or big changes in gene activity. They also observed that – as anticipated – spacing the editing out in time prevents the chromosomal chaos associated with making multiple DNA cuts at the same time.
“Immunotherapy is number one, two, and three on the list of applications,” says Nguyen. He envisions novel forms of CAR-T cells could be made to fight especially tricky cancers or to tame autoimmune diseases. Other future directions include combining peptides and antibodies to target CRISPR reagents to specific cell types.

Moving forward, the IGI Delivery Collective team is working to develop peptide-delivered CRISPR therapies that could be used in human patients. Without the need for specialized equipment, it might someday be possible to do genome editing in a hospital as a routine procedure. And because the peptide-CRISPR molecules are smaller than viruses or lipid nanoparticles, this approach may be especially effective for use directly inside a patient’s body, not just in isolated cells.
“Longer term down the road, this could offer a really exciting opportunity for point-of-care genome editing,” says co-first author Dana Foss, who led this research as a postdoc in the Wilson lab. “Right now that’s inaccessible because you have electroporation as part of a complicated workflow. But with the method we describe, it’s very possible since you just mix the peptide with the ready-to-go CRISPR proteins and add it to cells. The simplicity of the process opens up entirely new ways of getting cell therapy products to patients.”
Read more: Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes (Nature Biomedical Engineering, 25 April 2023)
 By
Hope Henderson
By
Hope Henderson