*See our updated 2024 clinical trials review.
Last year marked the 10th anniversary of the development of CRISPR as a genome-editing tool, an achievement that won Jennifer Dounda and Emmanuelle Charpentier the 2020 Nobel Prize in Chemistry. In the world of developing new medical therapies, 10 years is not a long time, but CRISPR-based therapies have made remarkable strides.

IGI’s Director of Technology and Translation, Fyodor Urnov, is excited about three big shifts in CRISPR-based medicines over the past year. The first is Intellia’s move from one liver-targeted therapy to another, changing only the guide RNA that dictates where in the genome the DNA edit will be made.

“In Jennifer and Emmanuelle’s 2012 paper, they said CRISPR is reprogrammable using simple rules,” says Urnov. “With Intellia this past year, we got the first formal evidence that this is true in the clinic. First they went after hATTR amyloidosis. And then they went after angioedema, using the same lipid nanoparticle, the same Cas9 mRNA, the same method of delivering the treatment with a single-dose IV infusion. They changed 20 nucleotides of the guideRNA and they got a medicine for a different disease. This is proving that CRISPR can be used as a therapeutic platform technology in the clinic.”
The second exciting shift is likely to come later this year. It is anticipated that Vertex and CRISPR Therapeutics’s collaboration on the CRISPR-based sickle cell disease treatment given to Victoria Gray and other trial participants in the US and Europe will be approved this year, likely first in Europe. This will be the first approval of a CRISPR-based therapy for use in humans and is a landmark moment in realizing the potential of CRISPR to improve human health. “This is not only the year where we’ve learned that CRISPR is a platform technology thanks to Intellia,” says Urnov, “But we’re also learning that this platform can be an approved medicine.”
The third shift is the growth of what Urnov calls “the clinical CRISPR ecosystem,” that is, the variety of different types of CRISPR-based genome editing that are being used in research and in patients. Recently, an individual was treated with base editing — a type of CRISPR-based editing that does not cause double-strand breaks in DNA and may be safer for use in humans — for the first time. Prime editing, another technique for edits without double-strand breaks, is also being developed for the clinic. Different types of CRISPR proteins, and CRISPR-based epigenome editors that alter how much protein is made from a given gene rather than changing the DNA sequence, are also being developed towards clinical use. These new technologies will expand the range of conditions that can potentially be treated using CRISPR tools.
The IGI closely tracks the development of new CRISPR-based therapies and the progress of the growing number of clinical trials. For 2023, our annual CRISPR clinical trials update focuses on what’s changed since our 2022 deep dive. Please refer to the 2022 CRISPR clinical trials article for disease background and an overview of how the clinical trial process works.
BLOOD DISORDERS
Sickle cell disease (SCD) and another genetic disorder affecting the hemoglobin in red blood cells, transfusion-dependent beta thalassemia (TBT), were among the first targets of CRISPR-based treatments. A number of groups have open trials using CRISPR-based treatments. Many of these take the approach of inducing expression of fetal hemoglobin (HbF), a kind of hemoglobin usually only found in fetuses and in very young infants. If the gene for fetal hemoglobin is turned “on” in adults, it can do the job of the healthy adult hemoglobin that individuals with SCD and beta thalassemia are missing.
ACTIVATING FETAL HEMOGLOBIN
The first CRISPR-based trial for SCD and TBT was sponsored by Vertex Pharmaceuticals and CRISPR Therapeutics. Their approach uses CRISPR to turn HbF back “on” in cells. The treatment is now in phase 3 trials, being assessed in both adults and children with severe SCD or TBT. They’ve shared results from over 40 individuals with TBT and over 30 with SCD: the results are dramatic and durable. 42 of 44 individuals with TBT were no longer transfusion dependent following the treatment, having been followed for up to three years. All of the participants with SCD are free of the vaso-occlusive crises that characterize the illness following treatment. Even without directly repairing the mutations that cause SCD or TBT, this treatment seems to be a functional cure for SCD and TBT. Hear from Victoria Gray, a participant with SCD who has experienced a remarkable recovery since undergoing treatment.
The treatment has been granted Regenerative Medicine Advanced Therapy, Fast Track, Orphan Drug, and Rare Pediatric Disease designations from the FDA for both SCD and TBT, and similar statuses from European Medicines Agency. Vertex and CRISPR Therapeutics have indicated they will seek approval in the US, EU, and UK this year. A phase 1/2 trial from Novartis and Intellia Therapeutics that takes the same approach should have data forthcoming, though the companies recently decided to discontinue their work on SCD.
Editas Medicine is also conducting a phase 1/2 trial for individuals with severe SCD, but using a CRISPR system with a Cas12a protein rather than the more famous Cas9 protein. Their approach, like that of the two trials described above, is to create edits that turn “on” HbF. This is the first time Cas12 has been used in a clinical trial, and it appears safe and without serious side effects in the first two trial participants. Early results for efficacy are positive as well, with neither patient experiencing vaso-occlusive crises as of December 2022, though this represents less than six months of follow-up. They are currently recruiting participants in the US and Canada.
In November 2022, Beam Therapeutics began enrolling participants in their US-based phase 1/2 trial of a base editing therapy for severe SCD, aimed at turning on HbF with single-letter changes to DNA. Base editing changes single DNA letters, or nucleotides, without creating double-stranded breaks in DNA, reducing certain safety risks. Beam is not expected to share data until 2024.
Read more:
- Vertex and CRISPR Therapeutics Present New Data on More Patients With Longer Follow-Up Treated With exagamglogene autotemcel (exa-cel) at the 2022 European Hematology Association (EHA) Congress
- Editas Medicine Announces Positive Safety And Efficacy Data From The First Two Patients Treated In The RUBY Trial Of EDIT-301 For The Treatment Of Severe Sickle Cell Disease
- Novartis data abstract — from the 2022 ASH conference
MUTATION CORRECTION
As of 2022, two groups were taking the alternative approach of directly correcting the disease-causing gene variant for SCD to the more common, healthy variant. In 2022, Graphite Bio dosed one participant in their phase 1/2 trial. Their approach combines electroporation to get the CRISPR proteins into the cell and a viral vector to get a DNA “template” that the new gene variant can be copied off of into the cell. In early January 2023, Graphite shared that the first participant is experiencing prolonged low blood cell counts (pancytopenia) requiring ongoing blood transfusions and other treatments. Graphite reported the event to the FDA and is investigating what happened. Initially, they decided to voluntarily pause the trial, but recently announced that they are discontinuing development of their SCD treatment.
A second trial, from a UC consortium including UCSF, UCLA, and the IGI, also aims to directly correct the disease-causing variant. While we don’t yet know what caused the negative outcome in the Graphite trial, one key difference between Graphite’s approach and the UC approach is that the UC approach does not use viral vectors. Enrollment in the UC trails has been delayed over the past year because of supply chain issues. The UC team expects to begin enrolling participants later in 2023.
Read more:
- More about the UC sickle cell trial
- Hit Pause: The Read-Across to Graphite Bio’s CEDAR Sickle Cell Trial Suspension
- Sickle cell pipeline narrows as gene therapy developers rethink research plans
A few very small trials (2–8) participants in China are looking at CRISPR treatments for TBT, but no data have been shared yet.
CANCERS
In CAR-T immunotherapy, researchers genetically engineer T cells to have a receptor that recognizes their cancer cells, telling the T cells to attack. The FDA has approved CAR-T made with traditional gene therapy in 2017, and it’s proven to be an effective approach. With CRISPR, researchers are hoping to make it even more powerful.
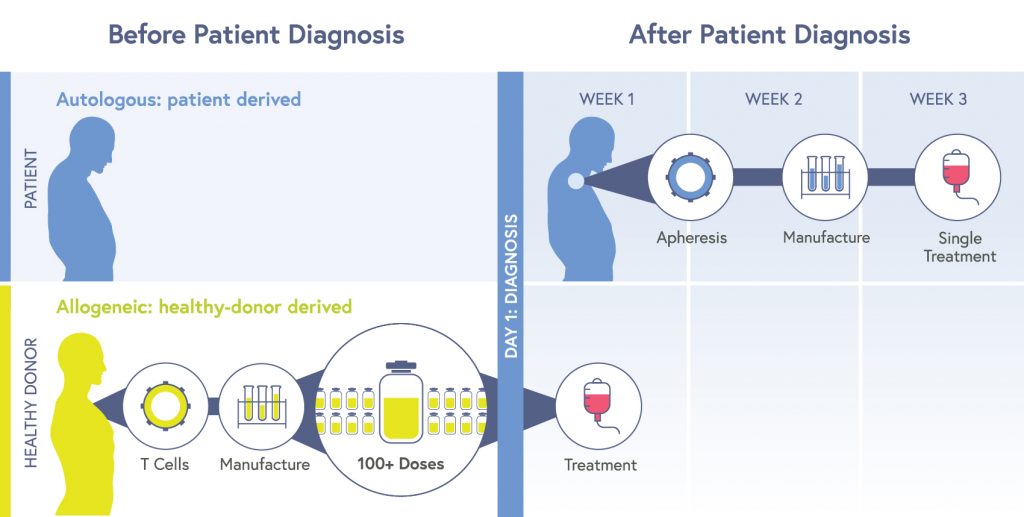
Most CAR-T therapies are autologous: cells are taken from an individual patient, edited, multiplied, and then put back into the same patient. Though the treatment is completed with a single dose, the process is expensive, time-consuming, and few facilities can do it. Sometimes the manufacturing process just doesn’t work, produces low potency cells, or individuals die of their disease while waiting for the manufacturing process to be completed.
A central focus for CRISPR researchers is making allogeneic CAR-T cells. Allogeneic therapies are made from cells from a healthy donor. These cells are edited to attack cancer cells and avoid being targeted by a recipient’s immune system or triggering graft-versus-host-disease, and then multiplied into huge batches which can be given to large numbers of recipients on demand. Allogeneic products reduce cost, time until treatment, and have the potential to provide consistently potent, high-quality cells.
LEUKEMIAS & LYMPHOMAS
CRISPR Therapeutics is testing two kinds of allogeneic CRISPR-modified CAR-T cells in phase 1 clinical trials. One targets CD70, a protein often present on the surface of cancerous cells in some lymphomas and some solid tumors. Last spring, CRISPR Therapeutics shared preliminary results for individuals with lymphomas, showing positive safety and tolerability results and a dose-dependent effect on disease activity. The treatment has been given the Regenerative Medicine Advanced Therapy (RMAT) designation by the FDA for certain lymphomas of the skin. This status is designed to speed up the approval of therapies addressing areas of unmet medical need.
The other treatment from CRISPR Therapeutics targets CD19, a protein often present on the surface of cancerous cells in leukemia and lymphoma. Read more about these types of cancers in last year’s clinical trials deep-dive.
In November 2022, CRISPR Therapeutics presented data showing a positive safety profile and revealed that about two-thirds of the patients responded to the treatment, with about 40% participants achieving complete remission (CR) in hard-to-treat, aggressive large B-cell lymphomas. 3/11 participants remain in CR as of two years of following treatment, and another 2/11 remain in CR as of one year following treatment. CRISPR-engineered allogeneic CAR-T cells. The treatment has also been given the RMAT designation.
Hear directly from patients who participated in the trial for this treatment:
Two other groups have had impressive results targeting CD19 for hard-to-treat, aggressive non-Hodgkin’s lymphomas. In December 2022, Caribou Biosciences released positive data on their US-based trial of allogeneic CRISPR-based CAR-T. Their cells, in addition to targeting CD19, have a second genetic modification: “knockout,” or genetic deactivation, of PD-1, a gene that cancer cells sometimes use to evade the immune system. The treatment was generally well-tolerated with an acceptable safety profile. In a US-based, phase 1 trial of 6 participants, all showed an initial total remission. After a year, 2/6 still show no sign of disease and are still being followed by the trial. Based on this initial data, the FDA has granted their product RMAT and Fast Track designations.
Bioray Laboratories in conjunction with Zhejiang University have also shared positive results from a phase 1 trial in China for a similar product. In their study, 7/8 participants observed for about a year had a complete remission and durable responses without serious adverse events. The other patient had a partial remission.
A team out of the UK’s NIHR Great Ormond Street Hospital Biomedical Research Centre shared results from a small phase 1 trial using CD19 targeting cells in children with B cell leukemias reached its safety endpoints. All together, this paints a promising picture for advances in treating blood cancers.
Read more:
- More about CAR-T targeting CD19
- More about effects of targeting CD70 in cutaneous lymphomas & the RMAT designation for this indication
- Caribou’s release of phase 1 data
- Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL — Nature paper with the Bioray study
- Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia — Science Translational Medicine paper on the study from the UK group
A big first for 2022, from the same Great Ormond Street group: CAR-T cells made with base editing, a kind of CRISPR-based editing that can make small changes to DNA without creating a double-stranded DNA break, was used for the first time. In May 2022, a teenager named Alyssa was treated with base-edited allogeneic CAR-T cells, achieving remission from acute lymphoblastic leukemia. Learn more about Alyssa’s story:
Read more:
- Tvt CAR7: Phase 1 Clinical Trial of Base-Edited “Universal” CAR7 T Cells for Paediatric Relapsed/Refractory T-ALL — Abstract from 2022 ASH meeting
SOLID TUMOR CANCERS
In November 2022, CRISPR Therapeutics shared preliminary results from a trial using their allogeneic CD70-targeting CAR-T cells in individuals with solid tumors in the kidney that are not being effectively controlled with standard therapies. The treatment was well-tolerated and without serious side effects. The treatment achieved a 77% disease control rate and at the last update, one participant no longer showed any signs of disease. CRISPR Therapeutics is developing a new version of the therapy with edits to additional genomic targets.
PACT Pharma did a US-based phase 1 trial for metastasized bladder, lung, head and neck, colorectal, ovarian, breast, and prostate cancers. Their approach is to analyze an individual tumor genome, and then use CRISPR to make an individual’s T cells target their specific tumor. Up to three different engineered T cells were given to each patient in their single-dose treatment. In November 2022, they shared data, stating that the gene-editing T cells preferentially infiltrated tumors, and led to tumor size reduction in one of 16 treated individuals. They have since suspended the trial as a “business decision.”
There are at least three other other phase 1 trials ongoing for solid cancers, including gastrointestinal, and epithelial-cell-derived cancers like breast and pancreatic cancers, but no results from these have been released yet.
Read more:
- CRISPR Therapeutics releases data on renal carcinoma treatment
- PACT Pharma Reports Data From First Clinical Study Using CRISPR to Substitute a Gene in Patients’ Immune Cells to Treat Cancer
- Non-viral precision T cell receptor replacement for personalized cell therapy — Nature paper with data from the PACT trial
GENETIC BLINDNESS
Leber congenital amaurosis (LCA) is the most common cause of inherited childhood blindness, and LCA10 is the most common form of LCA. This disease is caused by a single nucleotide change in a photoreceptor gene, leading to serious vision loss or blindness within the first few months of life.
LCA10 was the target of the first in vivo CRISPR therapy trial, held in the US and sponsored by Editas Medicine. The first patient was dosed in March 2020 and dosing of small cohorts continued through July 2022. Editas started with low-dose adult cohorts, and then moved on to high-dose adult cohorts as well as a pediatric cohort. This strategy was intended to reduce the risk of dangerous side effects throughout the trial and reduce risk to the pediatric cohort. Patients were dosed in a single eye, with the other eye serving as a control against which to test vision in the treated eye.
According to Editas press release materials, no serious adverse events or dose-limiting toxicities were observed. Treatment efficacy is tougher to evaluate than safety in these individuals. There was no way to directly assess what percentage of cells were edited or whether there were unwanted edits in participants. Because their vision is so reduced, the classic line-by-line letter reading eye test you may be familiar with cannot be used. A number of other tests, including mobility (e.g., ability to navigate around objects in one’s path) and ability to detect light were used.
Hear directly from trial participants Carlene Knight and Michael Kalberer:
In November 2020, Editas revealed that only three out of 14 patients had “clinically meaningful” changes to their vision. Two of the three responders had mutations in both of their copies of the relevant gene, suggesting that this treatment may be most effective in this subset of the LCA10 population.This subset of an already rare condition is represented by only about 300 individuals in the United States. Because the patient population is so small, Editas has decided to stop clinical development of this treatment. It may be resumed in future if they find an appropriate partner for this work.
Read more:
DIABETES
Researchers have long been interested in transplanting healthy pancreatic cells into individuals with type-1 diabetes (T1-D). While ongoing clinical trials in this area show that pancreatic cell transplantation can greatly benefit individuals with T1D, individuals who receive conventional pancreatic cell transplants must take drugs that suppress the immune system on an ongoing basis so that their body does not attack the transplanted cells. Immunosuppressant drugs can have serious side effects, including increased risk of dangerous infections and cancers.
Last year, we reported the start of a new clinical trial using pancreatic cells made from stem cells. CRISPR was used to edit the immune-related genes of these cells so that the patient’s immune system would not attack them. The cells were implanted into a patient’s body in a special pouch. Ideally, blood vessels would grow along the outside of the pouch, bringing the cells oxygen and vital nutrients from the blood, and taking up insulin from the cells. The aim was for patients to have healthy new pancreas cells to help control or even cure their T1D without having to take immunosuppressants. Stem-cell derived pancreatic cells also have a scalability advantage over conventional donor-matched or autologous transplants.
This phase 1 trial was sponsored by CRISPR Therapeutics and ViaCyte. and was the first use of CRISPR to treat an endocrine disease. However, shortly after the first patient was dosed in Spring 2022, ViaCyte was acquired by Vertex Pharmaceuticals. Vertex has their own ongoing trial of a stem cell-derived cell transplant product for T1D. It is not yet clear if they will pursue research with the intellectual property that they have acquired from ViaCyte.
Read more:
- Sweet Spot: CRISPR Therapeutics, ViaCyte Dose First Patient with Cell Therapy for Type 1 Diabetes — Includes more detailed information on the approach
- Vertex announces acquiring ViaCyte
CHRONIC INFECTION
Urinary tract infections (UTIs) are a common infection causing over 8 million visits to health care providers every year and are much more common in women. While most UTIs are easily treated with a short course of antibiotics, sometimes antibiotics are ineffective or the infection keeps recurring, leading to chronic UTIs.
The treatment currently in clinical trials is a cocktail of three bacteriophages combined with CRISPR-Cas3, designed to attack the genome of the three strains of E. coli responsible for about 95% of UTIs. The destruction of the genome kills the bacteria.
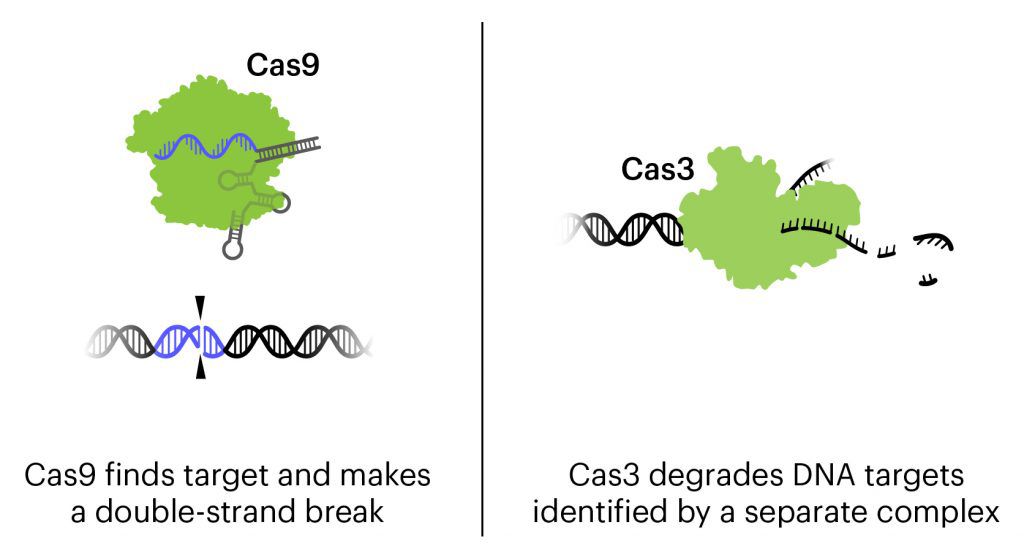
Bacteriophages, or phages for short, are viruses that attack bacteria. In this treatment, in addition to the natural action of phages that kills bacteria, the bacteriophages are engineered to contain CRISPR-Cas3 in their genome. Lesser known than its famous cousin Cas9, Cas3 shreds DNA at the gene regions it is targeted to find. In this treatment, the CRISPR-Cas3 system is made to target the genomes of the targeted E. coli strains. Locus Biosciences delivered the treatment directly to the bladder by catheter.
Locus Biosciences completed their US-based Phase 1b trial in February 2021. Locus’s trial was the first trial using a CRISPR-based therapy to treat infection. It is also the first trial to use the Cas3 protein, rather than Cas9. In press releases, Locus reported that results of the trial supported the safety and tolerability of the new therapy, with no drug-related adverse effects. No data have been published yet, but Locus says the initial results show a decrease in the level of E. coli in the bladder of participants given the CRISPR-based treatment.
Locus began enrolling participants for a phase 2/3 trial in 2022, and announced dosing of the first participant in September 2022. They plan to enroll approximately 800 participants from the US and European Union.
Read more:
PROTEIN-FOLDING DISEASE
Hereditary transthyretin amyloidosis (hATTR) is a fatal disease caused by mutations in a single DNA letter in the gene TTR, lead to a faulty version of the TTR protein. ATTR has similarities to other neurological diseases involving protein misfolding and amyloidosis including Alzheimer’s and Parkinson’s diseases. The treatment being investigated uses CRISPR-Cas9 tools to reduce the amount of faulty TTR protein the body makes and is delivered in a single dose by IV.
This is the first clinical trial for a CRISPR-Cas9 therapy delivered in a lipid nanoparticle (LNP). In animal models, LNPs have a tendency to accumulate in the liver. TTR is primarily made in the liver, so LNPs are a clever choice for getting the treatment to where it is needed. This is also the first trial to deliver genome-editing components systemically — that is, to the whole body rather than to one specific type of cell or tissue.
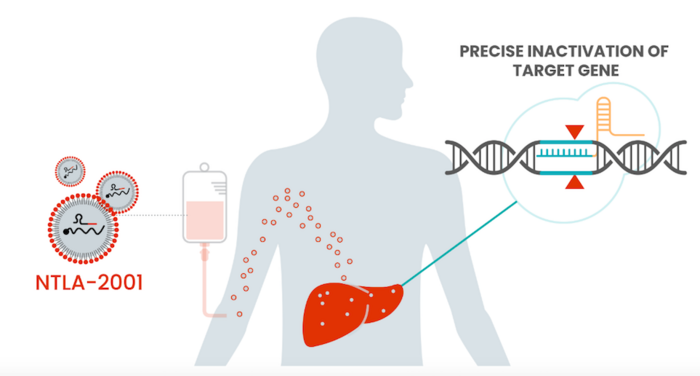
The trial, sponsored by Intellia with sites in the EU, UK, and New Zealand, has two arms and dosed the first participants in late 2020. One is studying patients with neuropathy symptoms and the other with cardiomyopathy symptoms. Between the two arms, data has been reported on 27 participants at a range of doses. Even at the lowest treatment dose, there is a deep (>85%) reduction in the amount of toxic protein in the participants’ blood streams, with greater than 90% reduction for participants receiving the highest dose. All patients are showing sustained reduction in the TTR protein over time, including the patients for whom a year of findings have been reported. As TTR protein levels correlate with disease severity, researchers are very optimistic about participant outcomes. Some side effects have been observed related to the infusion procedure, but were temporary and non-severe.
This year, Intellia intends to share additional trial data and initiate new trials including both patient groups, with participants in the US and around the globe. These trials will be aimed at collecting sufficient efficacy data for treatment approval by the FDA and other regulatory agencies.
Read more:
- CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis — Gillmore et al., New England Journal of Medicine
INFLAMMATORY DISEASE
In hereditary angioedema (HAE), an individual has severe attacks of inflammation, leading to swelling, often in the arms and legs, face, intestines, or airway. These attacks are painful and swelling of the airways can be life-threatening. Without treatment, attacks occur every 1–2 weeks, lasting 3–4 days each. HAE affects about 1 in every 100,000 people.
In healthy individuals, proteins that increase and decrease inflammation are in a careful balance, helping the body respond to threats and injuries to just the right degree. In some types of HAE, mutations lead to lower levels of the C1 inhibitor protein. This leads to increased inflammation and excessive fluid leak from the blood vessels into body tissue, and swelling episodes.
The treatment that is currently in clinical trials uses CRISPR-Cas9 tools to reduce the amount of an inflammatory protein the body makes. As in the hATTR treatment, the aim isn’t to fix a gene, but to break a gene to stop a disease process. As in hATTR, the liver is the main site of protein production, and Intellia again used lipid nanoparticles to deliver the therapy. This is an in vivo, systemic treatment, administered intravenously in a single dose.
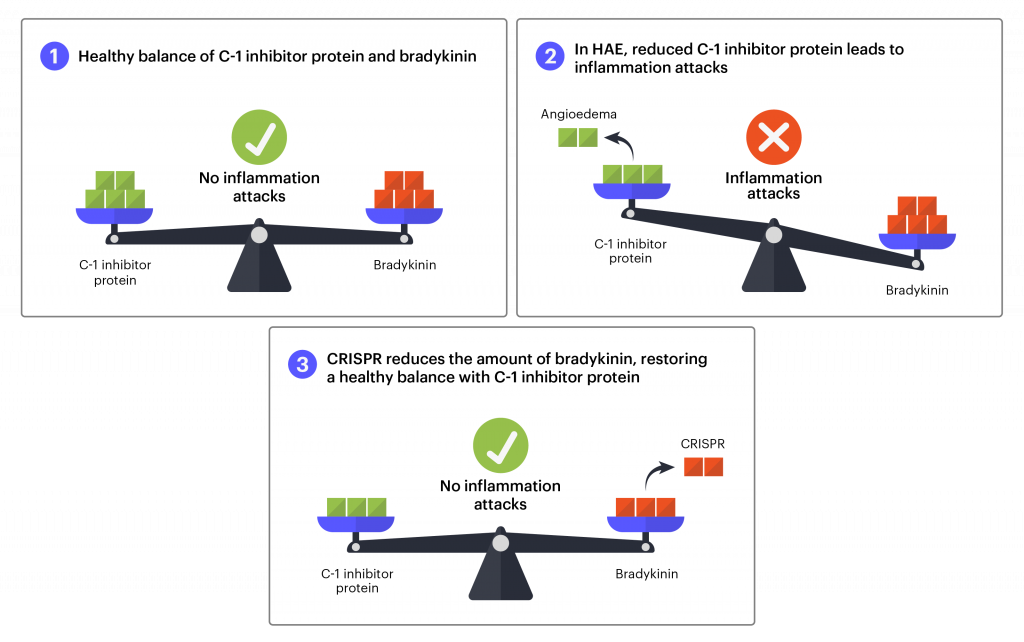
For HAE, researchers will use blood testing to see if the genome-editing components are successfully reducing the levels of proteins that cause inflammation as well as the number of inflammation attacks after treatment.
Early stage trials are designed to test safety and find the appropriate dosage of the treatment. Researchers tested a range of 3 doses on a total of ten participants in New Zealand. Most participants have been free from attacks in the interval between treatment and their most recent follow-up — as long as 10 months. Four months after treatment, there is a reduction in the amount of inflammatory protein of an average of 64% in the lowest dose group and over 90% in the highest dose group. The treatment has been well tolerated at all dose levels, with no severe adverse events. These results are extremely encouraging, suggesting the one-time treatment may represent a functional cure for this type of HAE. The US FDA has given the treatment orphan drug designation, a mechanism designed to financially incentivize the development of treatments for rare diseases. In the first half of 2023, Intellia expects to begin a Phase 2 dose-controlled trial with participants in New Zealand, the US, and other locations.
Read more:
CARDIOVASCULAR DISEASE
High levels of LDL cholesterol — so-called “bad cholesterol” — are a major risk factor for cardiovascular disease and premature death. Genetics play a role in cholesterol levels. Some individuals have mutations in the gene PCSK9 that leads to familial hypercholesterolemia: a heritable condition that causes dangerously high levels of cholesterol regardless of diet and exercise.
In 2022, Verve Therapeutics began their trial for a subtype of familial hypercholesterolemia using a base editor delivered by lipid nanoparticle (LNP). Base editing is a form of CRISPR-based editing that can make small changes to DNA sequences without making a double-stranded break in the DNA. Double-stranded breaks create unique risks to cells, and base editing, when applicable, may be a safer alternative to conventional CRISPR-based editing. In this case, the editor is designed to make a single letter change in the PCSK9 gene.
As in the case of hATTR and HAE, the liver is the major site of production of the protein in question. Lipid nanoparticles have a natural affinity for the liver, so LNP infusion is an obvious choice for delivery. Because the delivery is non-viral, the CRISPR components should only be present transiently, minimizing the risk of unwanted edits.
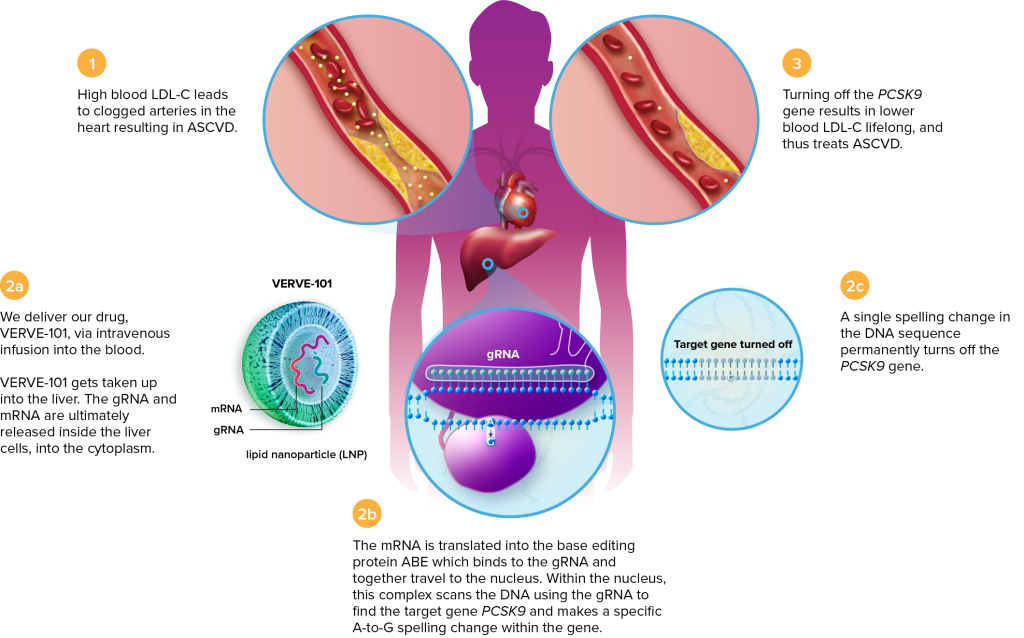
This phase 1 trial is recruiting participants in New Zealand and the United Kingdom. Verve anticipates enrolling up to 40 participants with a dose-escalation protocol. The first participant received the treatment in July 2022, and another two were dosed by October 2022. No serious side effects have been observed.
In the US, the FDA has put a hold on an IND that would allow a clinical trial of the therapeutic. The FDA has requested Verve provide more data on the treatment itself and on risks of editing in germline cells (e.g., sperm and eggs). The FDA wants to ensure that any DNA changes could not be passed down to future generations. Their requests do not stem from any concerning data released so far, but from theoretical safety concerns about this novel therapy.
Read more:
HIV/AIDS
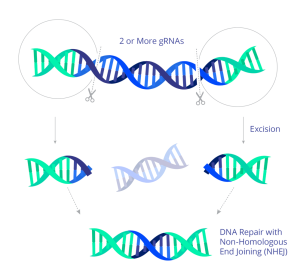
In 2022, the first participants were dosed in a US trial using CRISPR to treat HIV. The experimental treatment uses CRISPR genome-editing molecules to target the HIV DNA sequence stored in the host cell genome. In preclinical work, the guide RNAs direct the Cas9 protein to cut at two sites within the HIV genome, surgically excising most of the genome and effectively eliminating HIV from the cell. The CRISPR treatment is delivered by an AAV9 viral vector and administered by infusion.
The trial, which is sponsored by Excision Biotherapeutics, is the first to target HIV or any other chronic viral infection. The first participant was dosed in July 2022. As of September, there were no reports of significant severe adverse effects. This individual is expected to qualify for the next step in the trial, when they will be removed from standard antiretroviral therapy to evaluate the efficacy of the CRISPR treatment as a potential cure.
Read more:
MUSCULAR DYSTROPHY
Muscular dystrophies are a group of disorders that lead to muscle wasting and weakness. Duchenne Muscular Dystrophy (DMD) is the most severe form, leading to loss of voluntary movement, heart and lung failure, and death in young adulthood. DMD is an X-linked disorder, mostly affecting boys and men. A number of different mutations in the gene for the protein dystrophin can lead to DMD.
Biotech nonprofit Cure Rare Disease (CRD) created a personalized treatment for an individual with a deletion in the first part of the gene (exon 1). The aim of the treatment was to increase production of an alternate form of the dystrophin protein. This trial was designed to treat a single person — the brother of the CRD founder. The participant was dosed earlier this year at a US hospital and died sometime after the administration of the treatment. No information about the cause of this tragic death has been released yet. This is the first death associated with a CRISPR clinical trial, and it will be watched closely by the field.
Read more:
MORE INFORMATION ON CLINICAL TRIALS
If you or a loved one are interested in participating in a clinical trial, learn more about how US-based clinical trials work and where to find them on our Patients & Families page. Discuss all important medical decisions with your doctor. Keep in mind that clinical trials are the first tests of new medical treatments, so they are inherently risky and never guaranteed to be successful.
Thanks to Fyodor Urnov and Alex Marson for information and insights for this piece.
 By
Hope Henderson
By
Hope Henderson
Hope Henderson holds a B.A. in Biology from Brown University and a Ph.D. in Molecular & Cell Biology from the University of California, Berkeley. She joined the IGI in 2019 to work in science communication. In addition to serving as IGI’s main writer, she plans content strategy and manages IGI’s social media, illustration, and translation.