
2022 marked the 10th anniversary of the development of CRISPR as a genome-editing tool – and the first year that CRISPR-edited foods could be found on a grocery store shelf. In this article, we’ll go over the basics of genomic engineering in agriculture and then map out some of the most exciting new developments in 2022 from applications in crops and livestock. These include changing traits to adapt to a changing climate, improving taste or nutrition, protecting staple crops from disease, and more! We’ll also touch on the shifting regulatory landscape and what to watch for next.
ADDING CRISPR TO THE TOOLKIT
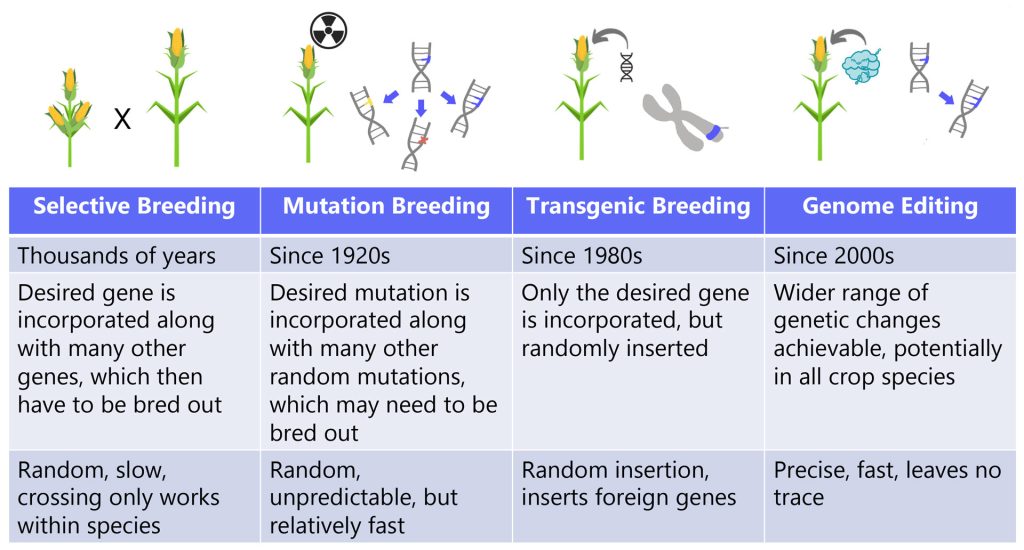
Relationships between humans, plants, and animals fundamentally changed with the introduction of agriculture, from exclusively making use of what was available in wild settings to intentional cultivation in novel settings. Farmers began selecting for crops and livestock with desirable traits like corn with many kernels or fruits with fewer toxins. They carried forward the organisms with these traits from generation to generation, in a process known as selective breeding.
Selective breeding was the first of many subsequent human interventions into agriculture, eventually complemented by newer techniques. For example, in crop plants techniques like cross-pollination, mutation breeding, transgenic breeding, and now gene editing are all in a lineage of tools with the goal of improving agriculture. Each of these approaches alters the genome – but CRISPR does it with unprecedented precision. From selective breeding to CRISPR we ask: How can we leverage these tools to generate crops and livestock that meet our needs?
In the news, you might hear about how CRISPR is already advancing human health: researchers are using CRISPR to delve deeper into the genetic basis of disease and CRISPR-based therapies are showing impressive results in clinical trials. Over the last decade, CRISPR has also been used to make genetic changes in a sweeping range of organisms beyond humans – including some of our most important and beloved crops and livestock.
CRISPR tools work similarly whether in a human, animal, or plant cell: they cut DNA at a specific site, programmed in by a researcher. This cut in the DNA signals the cell to recruit repair machinery. In the process of repairing the broken DNA, changes are sometimes made to the DNA. We can harness this process to develop new understandings of biological organisms, new cures for diseases, and new agricultural innovations. So far in agriculture, CRISPR is usually used to generate genetic “knockouts”: changes to genes that make them stop working.
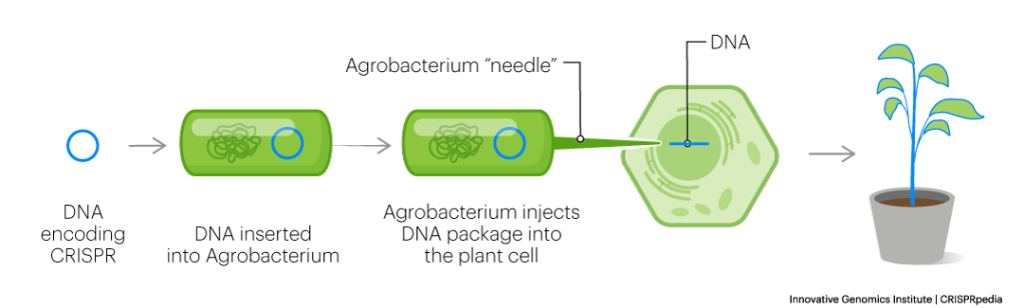
The big difference between animals and plants is how the gene-editing machinery is delivered into cells. In plants, two primary methods are used. In one, the bacterium Agrobacterium tumefaciens is used to transfer genes into plant genomes. In the other, gold-plated DNA is projected at plant tissues at high speeds and pressures to penetrate into the plant cell. Afterwards, the cells are grown into whole plants ready for research.
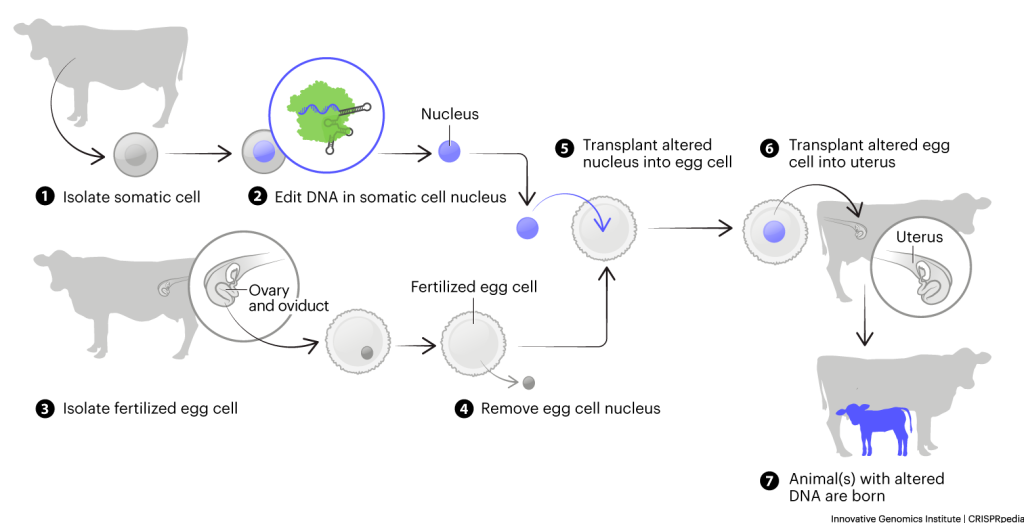
In fish, injecting gene-editing machinery directly into eggs is usually the most efficient way to produce gene-edited organisms. In mammals used in research and agriculture, two methods are used to generate edited embryos prior to transfer to a surrogate. In one, fertilized eggs are directly edited. The other uses somatic cell nuclear transfer, conventionally known as cloning. In this process, all the DNA in a fertilized egg is taken out and the DNA from an edited cell line is transferred in to make an gene-edited embryo. Note that these techniques are not currently being used in humans.
FROM LAB TO PLATE
Gene-edited crops and livestock start as an idea in a laboratory, where preliminary research reveals the potential of an edit to make a plant or animal with a desired trait. Promising results may be further supported by field trials, where plants are grown in contained settings more similar to the ones where they’ll be grown if successful.
In the United States, most gene-edited crops have genetic changes that could occur in nature and are exempted from regulation. Producers can request a confirmation from the USDA to ensure that the plant they developed meets the criteria for the exemption. Once a plant is given exempt status, it can be marketed, scaled, and distributed to growers and consumers alike. Depending on the specific changes, the FDA or EPA may also be involved in regulation. In the case of gene-edited animals, the FDA must approve the animal for commercialization.
Similar processes are in place all over the world with slight variations about what criteria must be met prior to an approval. Note that some countries require producers to prove that their product has the intended edit and does not contain any genes that come from a different organism. In 2022 a number of countries around the world decided to embrace gene-editing by providing pathways for exemption or approval of edited crops and livestock.
Once an animal or plant is reviewed by regulatory agencies and deemed safe, it can be marketed, scaled, and distributed to growers and consumers alike.
In this article, we break down the advances in CRISPR for agriculture into three major categories based on who primarily benefits — consumers, farmers, or the planet — with examples of interesting and notable products that are in the development pipeline. For each example, we denote where in the lab-to-field pipeline a gene-edited agricultural organism is.
GRAPHIC SUMMARY
Where are genome-edited products in the development pipeline? Hover over the icons to explore!
A graphical overview of the examples discussed in this article and their progress from research to commercial availability. Let your cursor hover over the icons for more info on each product. The microscope icon indicates products in the lab research stage; the wheat icon indicates products undergoing field trials; and the fork and knife indicate products that are commercialized. You’ll also see a checkmark symbol used in this article, indicating products given the green-light for commercialization.
EDITING FOR THE EATER: CONSUMER BENEFITS

Consumers – in this case, anyone who eats – have broad preferences and desires. Gene editing is a potent mechanism to deliver on these far-ranging food interests. Over the past year, researchers have used CRISPR to deliver nutritious leafy greens with milder flavor, long-lasting bananas, and tomatoes with higher GABA content. For the first time ever, food generated using CRISPR technology is being sold to consumers.
GABA-RICH TOMATO
Japanese food markets are world-renowned for the incredible quality of fish and huge array of rice varieties, among many other gleaming foodstuffs. They now have a new distinction: people in Japan are the first consumers in the world with access to gene-edited produce.
One of these options is the GABA tomato. Permitted by Japanese authorities in 2021, this tomato has found its way to thousands of home gardeners, farmers, and consumers in 2022. Produced by Tokyo-based Sanatech Seeds, these tomatoes have been edited to increase the accumulation of γ-aminobutyric acid or GABA. This compound is widely consumed as a supplement with claims of lowering blood pressure, improving mood, and increasing relaxation. While the clinical evidence supporting these claims is less than substantial, there is tremendous interest in GABA-enriched products in Japan. CRISPR-based editing of a single tomato gene produced lines with 4-20 times greater GABA levels. The verdict is still out whether GABA-rich tomatoes can provide real health benefits, but either way, being the first gene-edited product to hit supermarket shelves is a distinction!

MILDER MUSTARD GREENS

In the quest to assemble a gene-edited salad, one might add a GABA tomato to their cart alongside Conscious Greens. Recently unveiled by Pairwise, these greens have been gene edited to reduce the pungency of notoriously bitter mustard greens.
Using CRISPR, researchers at Pairwise transformed the nutritious but boldly-flavored leafy green into a milder salad green with the same nutritional profile. By editing a family of genes that catalyze the reaction known as the “mustard bomb,” these leafy greens have been disarmed into a more palatable form. Biochemical and sensory evaluations of these greens have validated that the source of the pungent flavors remains stably suppressed in fields and greenhouses.
Conscious Greens have recently made their way into the mouths and hearts of the public in various American cities where tasting events were hosted by the company. In 2023, they will be available in American grocery stores and restaurants.
NON-BROWNING BANANA

Few fruits are as ubiquitously loved and consumed as the banana. With a built-in wrapper and a sweet treat within, bananas have earned their spot as the most widely consumed fruit in the world.
Following the example of other non-browning products like the Arctic apple or the non-browning mushroom, Tropic Biosciences has achieved a similar type of product, this time using gene editing: a banana that can resist browning.

When exposed to oxygen, bananas and many other favorite foods like apples and avocados will start to brown. Thereactions that cause browning also usher in unpleasant flavors and odors, as well as reductions in nutritional quality. By removing the function of the enzyme that facilitates this reaction using CRISPR, bananas can stay yellow longer, appeasing consumers, and potentially reducing food waste. The USDA has given the banana the go-ahead for commercialization.
VITAMIN-D RICH TOMATO

You are what you eat, goes the old saying. Now, thanks to the work of scientists at the John Innes Center, we may be just a little bit healthier. In a recent study, a team of plant biologists successfully generated a tomato enriched with provitamin D3 using gene editing.
Vitamin D is crucial for bone health and regulating the immune system, and deficiency increases the risk of a range of health problems, including certain cancers, metabolic disorders, and worsened COVID-19 severity. Nearly 1 billion people have insufficient vitamin D levels. Food is the primary source of vitamin D and dietary interventions may be our best bet for addressing vitamin D deficiency.
Using CRISPR, scientists successfully removed the function of a gene responsible for converting 7-DHC, a vitamin D precursor, into the next compound in the biochemical process that eventually leads a plant to make vitamin D. Tomatoes with edits accumulated high levels of vitamin D precursor in leaves and fruit. These plants could then be exposed to UV light, in a process that mirrors sun exposure, to yield tomatoes with high levels of vitamin D. A single edited tomato can provide nearly 20% of the recommended daily allowance of vitamin D, with opportunities for greater accumulation possible still.
With nearly one-third of the global population affected by some type of micronutrient deficiency, this represents one of the most exciting uses of CRISPR in agriculture. Gene editing has the potential to provide solutions for a range of deficiencies beyond Vitamin D.
EDITING FOR A BOUNTIFUL HARVEST: HIGH YIELD

Whether it’s the Mid-Autumn festival or Sukkot, Mehregan, or the Andean Harvest Fiestas, nearly every culture in every corner of the Earth finds a way to honor the gifts of the harvest and to ask for abundant returns. Anxiety and celebration of crop and livestock yield is a thread woven through human experience that persists as threats to global food supply constantly emerge, alongside technological innovations like gene editing that could hold solutions.
In 2022, researchers worked on generating novel solutions to the evergreen issue of yield in agriculture. Some of these researchers made use of gene editing as a mechanism to achieve this end – with great success.
GROWING BIGGER FISH

To pair with the GABA-rich tomato, a Kyoto-based start-up, in collaboration with Kyoto and Kindai Universities has developed two gene-edited fish, red sea bream and tiger puffer, now available for purchase in Japan. Both fish were CRISPR-edited to grow larger.
In tiger puffer, gene editing was used to interrupt the function of genes associated with appetite regulation, leading the fish to eat more, ultimately increasing its growth rate and overall size. The edited tiger puffer weighs nearly twice as much as its conventional counterpart and can be market-ready in a shorter period of time.
The red sea bream is edited in the myostatin gene, which typically limits muscle development. Edited red sea bream fed the same quantity of food grew 1.2x larger than conventional sea bream. This same gene has been experimentally edited in sheep, cattle, pig, goat, rabbit, carp, and catfish, among other animals. Though successful in sea bream, editing this gene in other livestock sometimes causes health problems.

While these edited fish products may seem like something out of sci-fi, shortening time to market and increasing the amount of edible flesh per animal decreases the carbon footprint of raising livestock and increases the abundance of food available. Now available in Japanese markets, the tiger puffer and red sea bream alongside the GABA tomato comprise the three first commercially available gene-edited foods.
ANCESTRAL INSIGHTS TO MODERN YIELD INCREASES FOR MAIZE & RICE

Just a handful of crops occupy a grossly outsized proportion of our global calories. Despite hundreds of edible plant species, crops like maize, wheat, and rice comprise the majority of total crops consumed. Before they occupied such a gargantuan portion of our diets, these organisms didn’t look much different than the weedy grasses gardeners and farmers remove from their plots. The process by which these wild crop ancestors were transformed into the varieties planted globally is known as domestication. Selection for traits like larger and more numerous grains marked the genetic and aesthetic transition of these plants into the universally consumed and beloved foods that fuel us.
Researchers are able to study the genetic shifts that occurred in domestication by comparing the genomes of wild crop ancestors to those of modern varieties. By increasing our genetic understanding of this process, they are able to select gene targets for CRISPR-editing that could result in even greater yields.
One of these targets, KRN2, a gene involved in determining the size of the group of cells that will give rise to flowers and ultimately grains, was edited in rice and maize. This led to increasing yields by 8% and 10%, respectively. These increases represent major leaps forward especially when compared to near-stagnant yield improvements in conventional breeding programs. What’s more, the approach of looking to wild crop ancestors for a high-yielding path forward could be promising for other crop species as well.
| CRISPR-Combo for Newer, Faster Edits
An advancement in plant genome engineering was achieved this year by researchers at the University of Maryland. Scientists produced a new tool named CRISPR-Combo that works to simultaneously knockout target genes while activating others. Alternatively, CRISPR-Combo can be used to make precise changes to DNA using base editing — a kind of CRISPR-editing that can make tweaks to individual genetic letters without the need to break the DNA — while simultaneously activating other genes. A major bottleneck in plant gene editing is the lengthy process of forming a whole plant from an initial edited cell. CRISPR-Combo can be used to activate genes that help plants regenerate faster, as demonstrated in poplar, rice, and Arabidopsis thaliana, a plant commonly used in lab research. Excitingly, this tool can also increase the ease and speed of selecting plants that are transgene free, meaning that only the edits remain and no foreign DNA. This can help produce plants that can more easily pass through regulation. |
TEFF LUCK
The process of domestication was used on many crops to produce the modern varieties we grow today. Sometimes, the suite of traits typically associated with domestication – like larger fruits, more uniform time to maturity, and loss of toxic compounds – is only partially present in a given plant. These plants are known as semi-domesticated and have great potential for further improvement. This is the case for teff, a grain crop grown primarily in Ethiopia and Eritrea.

Teff is the most widely cultivated grain crop in Ethiopia. It is resistant to pests and diseases, and can grow in a wide range of environmental extremes unsuitable for other grass crops. However, this important grain crop still suffers from many disadvantages associated with undomesticated grasses. One of the primary limitations of higher-yielding teff production is its susceptibility to “lodging,” a process in which stems buckle under the weight of heavy grains near the top of the plant.
To overcome this limitation, researchers at the Donald Danforth Institute in collaboration with the Ethiopian Institute of Agricultural Research and Corteva Agrisciences are using gene editing to develop lodging-resistant teff. The gene target was derived from rice domestication – a natural precedent offering a roadmap for efficient gene editing. Knockouts of this gene produce teff that is able to remain upright, providing the potential to improve teff yields in the field. Excitingly, this example also represents one of the earliest demonstrations of CRISPR in teff and unveils great potential for applications of gene editing in crops that have regional significance.
CHANGING WHEAT FLOWERS TO INCREASE YEILD

Like its high-yielding, globally grown counterparts, maize and rice, wheat has been the subject of CRISPR-based yield improvements in the past year. The anatomy of flowers in grain crops has long been understood to be an important determinant of individual plant yield. By editing a gene associated with the development of flowers, researchers were able to markedly improve overall yield of wheat plants in field trials without any reductions of other important properties.
EDITING FOR A DYNAMIC PLANET:
CLIMATE & DISEASE RESISTANCE


Climate change is bringing more erratic rainfall, increased drought severity, warmer temperatures, and saltier soils, and these effects will intensify over the next decades. The scientific consensus: agricultural productivity will decline.
While the rate of climate change is unprecedented, what is not new is the ability of agricultural technology to respond to pressing demands. New varieties of crops are released consistently for traits that meet the moment, whether that be resistance to a papaya virus or a more nutritious sweet potato.
In a similar way, gene editing may offer another tool to address the severe effects climate change is already starting to have on agriculture.
MOOOOO-RE HEAT TOLERANCE

The United States FDA made the determination that a genome-edited beef cattle with a short coat can be commercialized. The decision provided to Acceligen Inc., the developer of this cow, certifies that animals with this edit do not raise any safety concerns. This is the first time the FDA has given a green light on a gene-edited animal intended for human consumption, paving a path forward for other gene-edited animals.
Beyond the novelty of being permitted, what is so special about these cattle, anyways? Warming temperatures cause physiological stress to cattle: their body temperature increases, their breathing speeds up, and in dairy cows, milk production goes down. The cattle in this example have an edit that gives them short-hair, also known as a “slick” coat. Shorter hair means that these Travolta-esque slick cows can stay cooler in warm temperatures, increasing animal welfare and ultimately beef production in a changing climate.

The changes made by gene editing resemble naturally occurring variations in the same gene often found in tropical cattle breeds. Gene editing enables the transfer of this short-haired trait into good meat-producing breeds in a streamlined manner. In other words, CRISPR is giving overheated cows a genetic haircut and the ability to yield well despite a warming world.
WHEAT DISEASE-RESISTANCE
Removing the function of genes by gene editing can sometimes deliver desirable results like high-yielding wheat or tastier greens. However, sometimes the genes that control traits of interest have more than one job. This is especially true for genes known as “susceptibility genes”. Disrupting the function of disease susceptibility genes can give crops resistance to diseases but often also results in lower yield.
Researchers interested in making wheat that is resistant to powdery mildew but could produce normal amounts of grain were challenged to find a new way forward. In an exciting new study, they were able to knock out a susceptibility gene alongside an additional edit thought to affect yield. The susceptibility gene targeted in this study is one that has been edited by itself in tomato, grape, and wheat. These two edits had never been done together before, and together generated powdery mildew-resistant wheat with no yield penalties. Beyond providing a new type of resistant, high-yielding wheat, this study indicates that susceptibility gene editing doesn’t always have to mean lower yield.
As the climate changes, it is expected that infectious diseases will get more severe. Changes to the climate can create environments that are more favorable for pathogens and for the vectors, like insects, that help spread those pathogens. This study shows that when strategically applied, gene editing may help plants and animals resist diseases – without yield losses.
STUDYING A PIG VIRUS WITH CRISPR

Senecavirus A (SVA) is an increasingly damaging disease in the swine industry. Increasing understanding of how this virus works in pigs could help researchers develop strategies for managing its spread.
In a recent study, CRISPR was used to remove the function of a pig gene named ANTXR1. This gene was thought to be a receptor for SVA in pigs. Indeed, disrupting the ANTXR1 gene using CRISPR resulted in pigs that had dramatically lower levels of virus. Unfortunately, pigs with this edit had other undesired attributes, such as cataracts at birth.
In this case, simply removing the function of this gene is not a viable option for preventing infection. The improved understanding of viral infection facilitated via CRISPR may enable the development of new strategies for virus mitigation in swine.
THE BIG PICTURE
This year, for the first time ever, multiple gene-edited foods were available to the public! Landing on the shelves of Japanese shoppers were gene-edited red sea bream, puffer fish, and GABA-rich tomatoes. New varieties and breeds permitted by the USDA and FDA, like the milder mustard greens and the heat-tolerant cow, will likely be available to consumers in the coming months or years.

We can expect more products to arrive in grocery stores as different countries around the globe outline clear paths to commercialization. India, Kenya, and thePhilippines joined a growing list of countries to develop regulation regarding gene-edited crops this year. Without clear regulation, gene-edited products cannot be legally commercialized. These new policies are a significant step towards expanding the global presence of gene-edited foodstuffs by offering a pipeline to commercialization. Over the last couple decades, the regulation of GMO products was largely determined by movements in Europe. In contrast, new regulations on gene-edited products from Asia and Africa signal a shift in power towards greater market autonomy.
Beyond new enabling policy, technological advances stand to accelerate the development of new gene-edited crops and livestock. Currently, gene editing is largely used to make gene “knockouts”. The emergence of technology like CRISPR-Combo that can help researchers more effectively make other kinds of genetic changes like turning a gene “up” or adding new DNA sequences, gets us closer to ultimately leveraging the full, versatile capacity of CRISPR.
WHAT TO WATCH FOR
Looking forward, we can expect that CRISPR-edited crops and livestock will continue to emerge in the literature, the lab, and even in our markets over the next couple years, aimed at traits related to climate adaptation, consumer quality improvement, and yield enhancement. Keep an eye out for gene edits that go beyond the simple “knockout” in favor of precise gene insertions, base edits, or multiple types of edits simultaneously: these will represent growing use of more technically complex edits. The European Union, which has some of the most restrictive regulation of gene-edited products, has indicated that they’re working on a fast-track approval process for crops that are edited for sustainability. We also expect updates to US policies and that nations in South America, Asia, and Africa will continue to develop new gene-editing regulations.
The technological power of CRISPR is uncontested. But the ultimate impact remains to be seen: impact depends on implementation, which includes favorable regulation, grower education, public understanding and acceptance. It is not just technological innovation, but navigating the complex sociopolitical landscape of food systems, that is crucial to how – and how much – gene editing takes root as a mainstay of our food system.




