CRISPR in Agriculture

Introduction
HOW WILL CRISPR TOOLS HELP FEED FUTURE GENERATIONS?
CRISPR tools will have major impacts on farming. This CRISPRpedia chapter describes how CRISPR tools can be used to breed plants and animals with useful traits. You’ll also learn about the difference between genome-edited and “GM” plants and animals.
Engineering plants
The many methods to grow plants with desired traits
VOCABULARY
Selective breeding, mutation, mutagenesis, transgenic, genetically modified/GM/GMO
SELECTIVE BREEDING
Plant genetic modification efforts — to improve flavor, yield, and more — began thousands of years before people knew what DNA was. For example, to develop modern corn, ancient farmers in what is today Mexico selectively cultivated rare variants of teosinte, a skinnier, grassier plant. Their efforts may have looked something like this:
- They planted lots of teosinte for its seeds
- They noticed a few rare plants with qualities they enjoyed, like bigger, juicier kernels
- They bred and planted seeds from the plants with the desired trait, increasing the frequency of the trait in the population
- They repeated this process when they found more plants with beneficial traits, like a higher number of kernels per plant, resulting in the crop we recognize as corn
This process is called selective breeding. It relies on the natural emergence of variants with a desired trait, resulting from occasional random DNA changes, or mutations, that naturally occur during reproduction. Over decades or centuries, farmers keep and mix these rare, desired traits together through mating and produce plants with the characteristics they want. Remember that DNA encodes the proteins that give plants their specific characteristics: by selectively breeding to change traits, farmers were actually selecting and breeding plants with the DNA variants they wanted.
Selective breeding has been hugely important in the history of human civilization, creating crops with higher yield, improved nutrition, and making farming more efficient. Crops created with selective breeding also tend to have been bred to taste less bitter and more sweet than their wild counterparts, appealing to our taste buds.
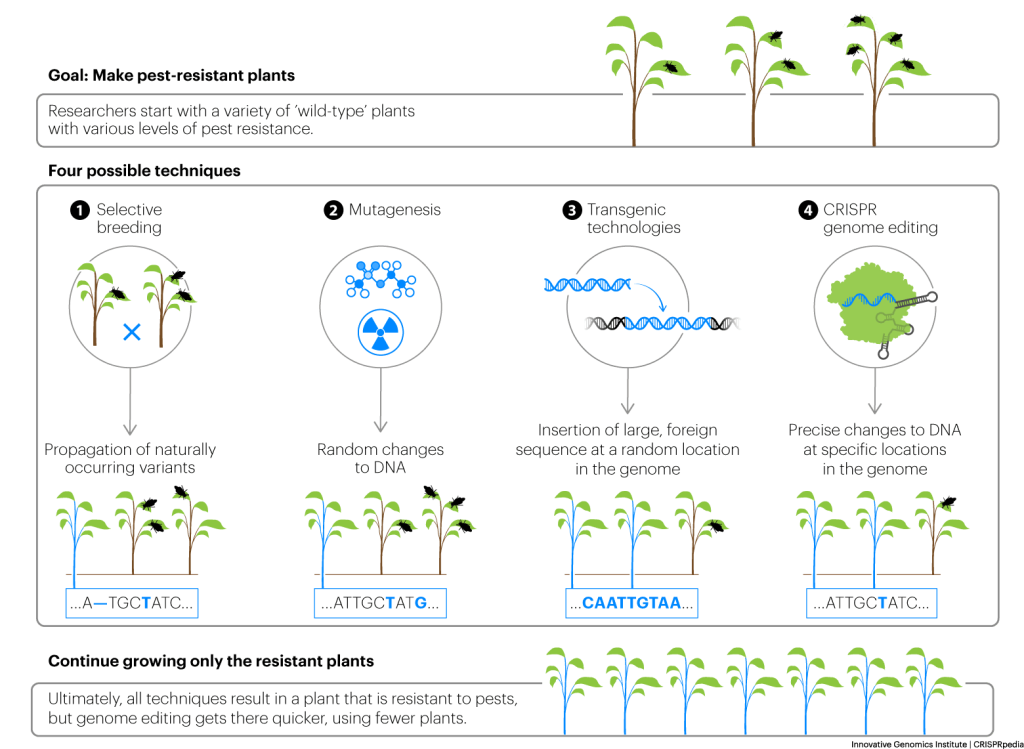
MUTAGENESIS/MUTATION BREEDING
DNA changes that lead to visible trait changes occur naturally, but rarely. Plant breeders have also used mutation breeding or mutagenesis — techniques that create a high number of random DNA changes — to make them appear more quickly. This works by exposing plants to radiation or applying mutagenic chemicals that cause random DNA changes. Some of the DNA variants will have no visible effect on the organism. Some will lead to new but undesirable traits. And some will lead new desirable traits. Researchers sift through thousands of plants to find the individuals with desired traits. As in conventional selective breeding, these individuals are bred and propagated until researchers have a strain that reliably shows the desired trait. One well known example is the ruby red grapefruit, which was developed using radiation mutagenesis in the 1970s.
Mutation breeding is faster than selective breeding, but like selective breeding, mutation breeding relies on finding a desired trait created by random changes. Selective breeding and DNA mutagenesis techniques are time-consuming and laborious. And sometimes random undesired changes come along with the desired changes when genes or other DNA sequences important for a given trait are close to each other in the DNA. For example, researchers have found that a DNA sequence important for wheat plant height is next to another DNA sequence important for kernel size. If they have a tall plant with small kernels, it can be very challenging to get a tall plant with big kernels.
BEYOND RANDOM VARIATION
Genome engineering approaches – that is, making targeted, instead of random, changes to DNA – can massively speed up the process of producing plants with desired traits.
TRANSGENICS/GMOS
To make plants with desired traits in a targeted way, plant breeders can use a variety of genetic modification techniques to make intentional alterations to plant DNA. They may use these techniques to change, delete, or add proteins. These proteins carry out cellular functions that result in the traits they’re interested in changing.
In the recent past, using these classic genetic modification techniques, it was much easier to add new DNA sequences than to edit existing DNA. These modified plants with new sequences usually contained some DNA sequences from other organisms, sometimes referred to as “foreign DNA”. An organism with DNA from another organism is considered transgenic. Researchers can make transgenic plants quickly and they usually come with fewer unintended traits than plant variants created using methods that rely on random DNA changes.

| You may have heard of ‘insect-resistant plants.’ These crop plants contain genes from bacteria (“Bt genes”) that make them toxic to insects, but not to humans. When growing Bt crops, farmers no longer need to use conventional pesticides, which can be costly, toxic for farmworkers to inhale or handle, and pollute nearby soil and water.
When people talk about genetically modified plants or GM crops or GMOstoday, they are usually talking about transgenics. Although years of scientific research shows that transgenic crops currently on the market are safe to eat. However, transgenics have struggled to gain popularity with consumers: some people worry about their food safety or object to the idea of moving genes from one species to another. From a developer or farmer’s perspective, the practical result is that many countries regulate transgenic plants more strictly than non-transgenic plants. Transgenic plant producers must spend a lot of money and time to show that their products are safe and risk consumer backlash with or without this data.
USING CRISPR TO CREATE PLANTS WITH SPECIFIC TRAITS
Researchers can use CRISPR genome editing to create plants with specific, desired traits. Researchers use CRISPR to precisely alter plant DNA sequences known to be related to particular traits, in order to breed plants with desired traits.
Because they are so precise, CRISPR tools avoid many of the negative side-effects of older plant breeding techniques. CRISPR tools can be used to make changes to plant DNA without adding any foreign DNA. Instead, they can be used to make small edits to the DNA already in plants. Regulators place less stringent regulations on these non-transgenic, genome-edited plants because they have DNA sequences that could occur naturally: CRISPR tools just speed up the process and add precision.
Despite the usefulness of CRISPR tools, they cannot yet efficiently replace older transgenic tools, e.g. the tools that were used to create Bt crops. For now, in addition to breeding approaches, both CRISPR and older transgenic tools will continue to be important in crop genetic modification. As CRISPR tools continue to be developed and refined, they may take the place of older tools in creating transgenic crops.
Delivering CRISPR to plants
Researchers can get CRISPR tools into plant cells by using bacteria, breaking down plant cell walls, or shooting DNA into plant cells
VOCABULARY
Delivery, Agrobacteria, protoplast, biolistic delivery, gene gun, nanotube
Researchers use CRISPR genome-edited plants to learn about plant biology and solve agricultural problems. The processes researchers use to create genome-edited plants are a little different than those used for creating genome-edited microbes, animal cells, or animals. A major reason for this is that plants have cell walls that can be difficult to bypass. Because of this, developing protocols to deliver CRISPR genome-editing tools to different plant species takes time.
Researchers deliver CRISPR tools to plants using three main methods:
- Agrobacteria delivery
- Protoplast delivery
- Biolistic delivery/gene gun
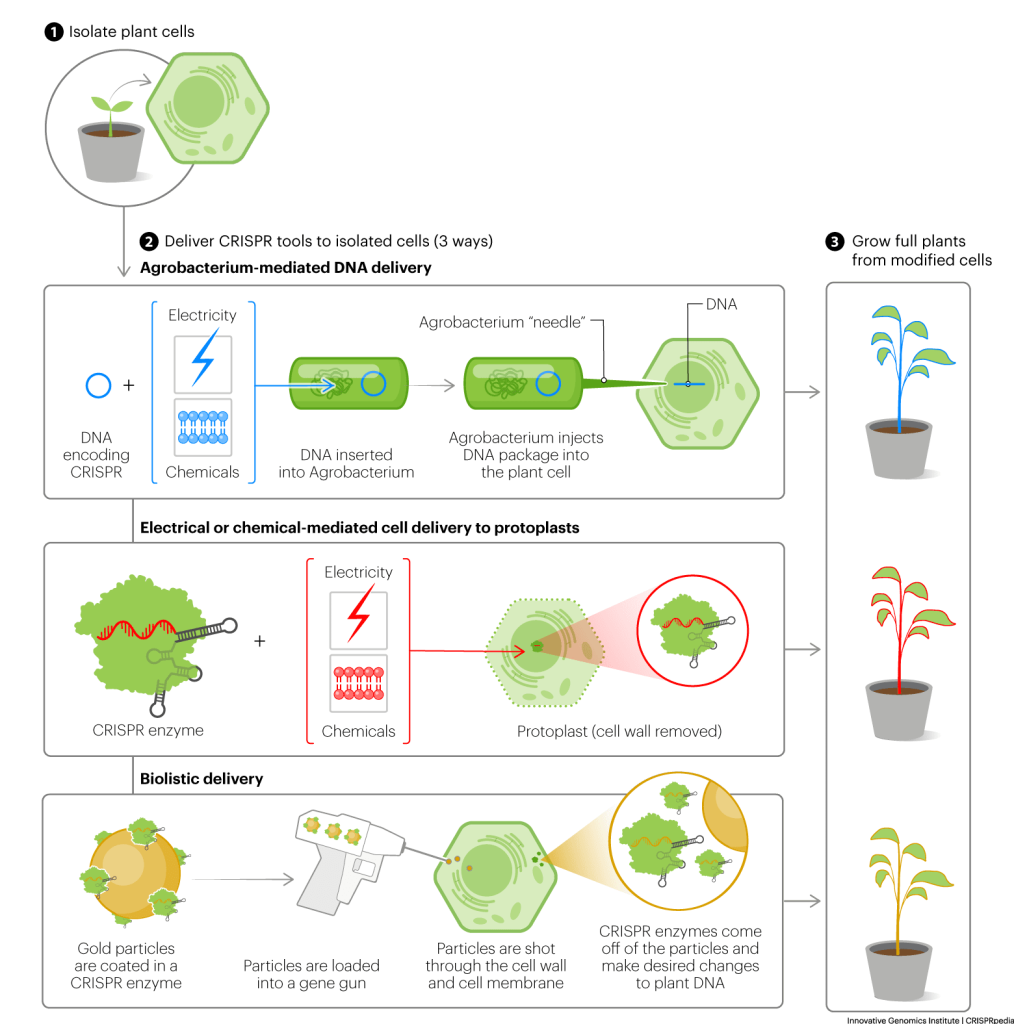
AGROBACTERIA DELIVERY
In this method, researchers use specific bacteria, Agrobacteria, as “delivery people.” First, researchers encode CRISPR tools in special, circular pieces of DNA. These are the “packages” that the Agrobacteria will deliver. Next, they use chemical or electrical shock to bypass the plant’s cell wall and put these circular pieces of DNA into the agrobacteria. Then they grow the Agrobacteria alongside plant cells.
As they grow, the Agrobacteria create tiny, protein-based needles. They use these needles to inject the DNA packages into plant cells. The plants cells read this DNA and make the encoded CRISPR tools. The tools then get to work editing plant DNA.
This method works with many different plants, but it does have a few drawbacks. In particular, the injected DNA inserts into random locations in the plant genome. So, the encoded CRISPR tools stick around after they’ve made the desired DNA changes. The longer the tools stick around, the more likely they are to make unwanted edits. These plants are also considered transgenic, since the DNA encoding CRISPR tools is derived from bacteria.
Researchers can use additional techniques to remove the CRISPR tools from their edited plants. For example, they can breed their altered plants with unaltered plants. Some of the resulting offspring will inherit only the DNA with the desired changes and not the CRISPR tools. These techniques are effective but take significant effort and time.
PROTOPLAST DELIVERY
Like castle walls, plant cell walls protect plants and provide them with structure. They also prevent scientists from using brute force – chemical and electrical shock – to get DNA into plant cells. So researchers have developed ways to get rid of plant cell walls. Scientists call the resulting, wall-less plant cells protoplasts. Scientists can much more easily deliver CRISPR tools to protoplasts. These tools then alter or edit the protoplast DNA. After the desired changes are made, scientists can sometimes grow full plants from the altered protoplasts, although this remains quite challenging for most plants.
With protoplast delivery, scientists can deliver DNA encoding CRISPR tools, but they can also deliver ready-to-use CRISPR enzymes and RNA guide molecules. These ready-to-use tools edit plant DNA but don’t insert into it. This spares researchers having to take extra steps to remove these tools from their altered plants later.
Protoplast delivery is effective, but it’s currently difficult to recreate full plants from protoplasts. This method is not always workable as a method to breed plants, but it is an efficient way to study different genome changes in a lab setting.
BIOLISTIC DELIVERY/GENE GUN
In biolistic delivery, researchers literally shoot CRISPR tools into plant cells using gene guns. Researchers load gene guns with tiny, CRISPR tool-coated gold particles. These tools can be in the form of DNA, RNA, and/or ready-to-use CRISPR enzymes. Then they fire the particles at plant cells. The particles travel straight through plant membranes and cell walls. The CRISPR tools then come off the particles and get to work editing plant DNA.
Biolistic methods can be used for many types of plants. However, if researchers deliver DNA with biolistic methods, things get a bit messy. Plant cells can insert many copies of the delivered DNA into their own DNA. This can result in useless or detrimental production of the encoded CRISPR tools. It can also make the tools difficult to remove later.
THE FUTURE OF CRISPR DELIVERY TO PLANTS
Beyond these more common methods, scientists are developing other methods including nanotubes as delivery vehicles. Nanotubes are tiny carbon-based structures that scientists can coat with ready-to-use CRISPR tools. When grown alongside plant cells, these tubes puncture the cells. The CRISPR tools then travel from the tubes into the plants cells and start editing plant DNA. As researchers refine their nanotubes, perhaps they’ll usurp the techniques above — we’ll have to wait and see!
Applications of CRISPR in plants
CRISPR tools could be used in research, nutrition, biomanufacturing, conservation, and more
VOCABULARY
Biomass, genetic containment, vegetative propagation
CHANGING TRAITS WITH CRISPR TOOLS
CRISPR genome-editing tools have many applications in plants: researchers use them to learn more about plant biology. Plant breeders use them to create crops with desirable traits. Scientists can even use them to turn plants into leafy manufacturing facilities. Researchers are continually finding new applications of gene editing in plants, many of which go well beyond agriculture. What other applications can you imagine?
CRISPR TOOLS IN PLANT RESEARCH
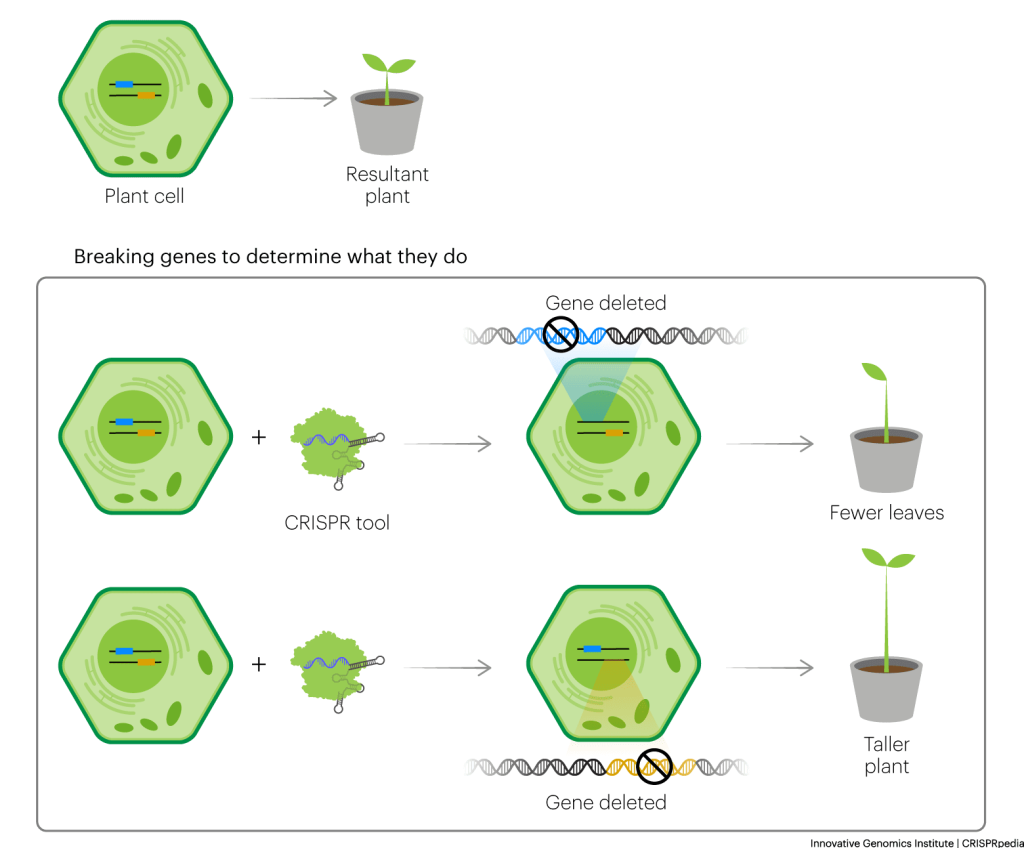
DNA sequences encode the proteins that give plants their specific traits. Farmers have been selectively breeding plants to get desired traits for thousands of years, but we still have a lot to learn about plant genetics. Scientists often know of a few DNA sequences associated with particular plant traits, but they might not have a precise understanding of what individual DNA sequences do or what other genes or sequences might also be involved. CRISPR tools can help them figure this out.
For example, with CRISPR tools researchers can “break” plant DNA sequences. Then they can see how the break affects traits they’re interested in. This helps reveal the functions of the parts encoded by the broken DNA. Refer to “Breaking Genes to Learn About What They Do” in the CRISPR Technology chapter to review this concept in depth.
IMPROVING GROWTH UNDER STRESSFUL CONDITIONS
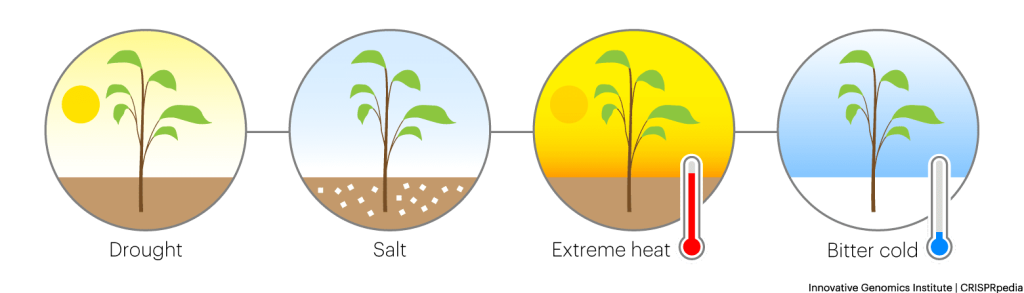
The world population is growing. At the same time, climate change is making it more difficult to grow many important food crops. So, researchers and farmers need to figure out how to adapt agriculture to changing, and sometimes increasingly harsh environmental conditions.
Researchers are actively working on this, and have discovered some DNA variants that increase crop resilience to extreme environments, including drought. They are using CRISPR tools to make these changes in important staple crops like corn, wheat, rice, and sugarcane. Some of these crops are currently being tested in the field, with more to come.
IMPROVING PEST AND DISEASE RESISTANCE

Like us, plants get infections. They can also be eaten by pests. Together, these cause huge crop and revenue loss for farmers. They can even wipe out entire plant varieties. Genome-editing technology is one tool that farmers can use to fight pests and plant diseases.
Farmers have been growing insect-resistant genetically-modified crops for many years. Often, these crops produce bacterial proteins that kill or repel insects, preventing the crops from being eaten by insect pests. ‘BT crops’ are engineered to produce a toxin from the bacterium Bacillus thuringiensis, a natural insecticide that protects crops from pests but is not harmful to humans.
Farmers also grow herbicide-resistant GM crops. These crops produce bacterial proteins that make them resistant to chemicals that kill plants. This allows farmers to use herbicides to kill weeds without harming their crops. The traits in these GM crops come from the addition of non-plant, bacterial DNA to plants. In other words, they are transgenics.
CRISPR genome editing can sometimes create plants with similar traits by targeted editing of the plant’s own DNA, rather than by addition of non-plant DNA. For example, many viruses need plant proteins to grow, multiply, and spread. Researchers can use CRISPR tools to break or change the plant proteins a particular virus likes to take advantage of. As a result, the viruses won’t be able to spread within the edited plants. Similar techniques can make plants resistant to harmful bacteria, fungi, and insects.
Do CRISPR tools make GM plants?
CRISPR is a tool. Whether it makes genome-edited or transgenic/GM plants depends on how it is used. If it is used to edit the DNA that a plant naturally has, the resulting plant is not transgenic/GM. If it is used to add DNA from a different species, then the resulting plant is transgenic/GM.
For example, researchers are working on using CRISPR tools to boost plants’ natural defenses, through both non-transgenic and transgenic approaches. Like humans, plants have immune systems that protect them from disease-causing microorganisms. CRISPR tools can be used to “turn up” plant immune defenses by making edits to the DNA sequences that are already in the plant (non-transgenic). They can also shuffle defense proteins between different species of plants (transgenic) or even insert new defense systems from other organisms into plants (transgenic).
IMPROVING COMMERCIAL PROPERTIES
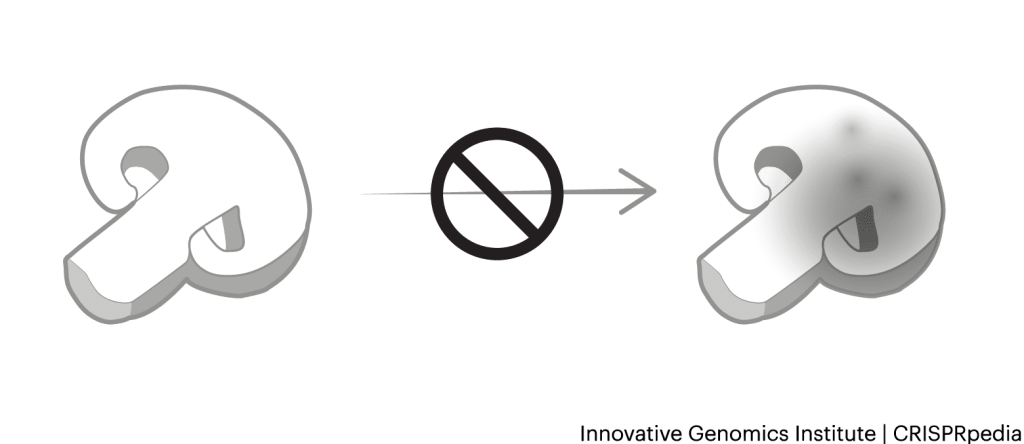
Many of the plants we have today were made with selective breeding and DNA mutagenesis techniques, but these sometimes come with negative side effects. These side effects happen when traits you don’t want come along with the ones you do want. On a molecular level, this can happen because DNA for one trait is next to DNA for another, so they get passed down together.
One example of negative side effects is in many of the varieties of big red tomatoes at the supermarket. Farmers bred these tomatoes to be beautiful and transport without turning to mush, but they’ve lost much of their flavor. More delicate heirloom tomato varieties have sweeter, brighter flavors, but don’t transport well.
Researchers may be able to use CRISPR tools to correct these negative side effects, improving crops in ways that make them more appealing to consumers. For example, researchers recently identified DNA sequences responsible for pleasing tomato flavors. With CRISPR tools, they might be able edit or restore these sequences, making more flavorful versions of large supermarket tomatoes.
Some other ways CRISPR tools could change fruits and vegetable traits include:
- Preventing bruising
- Increasing shelf life
- Creating seedless fruits
- Changing color/appearance/flavor
- Improving nutrient profile
- Decaffeinating coffee
Beyond these consumer-friendly improvements — or unfriendly, depending on how you like your coffee — researchers can also make plants easier to farm. For example, they can change stalk heights to prevent crops from falling over or prevent plants from scattering their seeds.
MAKING PLANTS MORE PRODUCTIVE
Plants use photosynthesis to convert sunlight into energy that helps them grow, creating the stems, leaves, seeds, and fruits that make up their biomass. To produce food and raw materials for the world’s growing population, farmers need ways to grow more plant biomass. And to protect our natural environment, they must do so using fewer resources.
Different kinds of plants have different photosynthetic systems. Some bacteria also have their own photosynthetic systems, and some of these systems are more efficient at converting sunlight into energy and biomass than others. Researchers are using CRISPR tools to edit the proteins involved in photosynthesis to produce plants that are better at converting sunlight into biomass, helping farmers feed the world.
IMPROVING NUTRITION
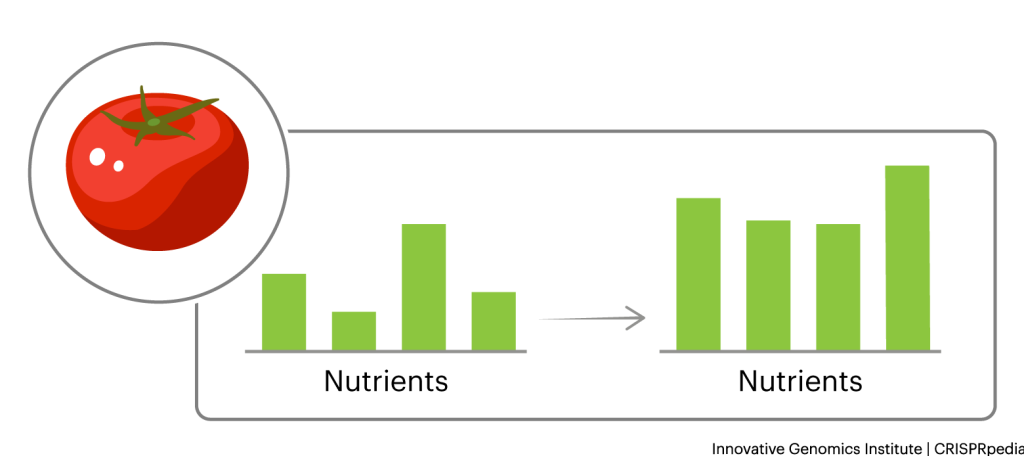
People all over the world suffer from poor nutrition. Researchers can use CRISPR tools to create more nutritious plant varieties. For example, they can make some plants produce more vitamins, like so-called “golden bananas” that have a richer color due to their increased beta carotene. They can make some plants easier for humans and other animals to digest. They can even remove allergens and toxins; for example, researchers are working on removing allergenic proteins from peanuts and cyanide from cassava. If used for these ends, CRISPR tools could make more nutritious, safe foods available to more people.
TURNING PLANTS INTO BIOLOGICAL FACTORIES
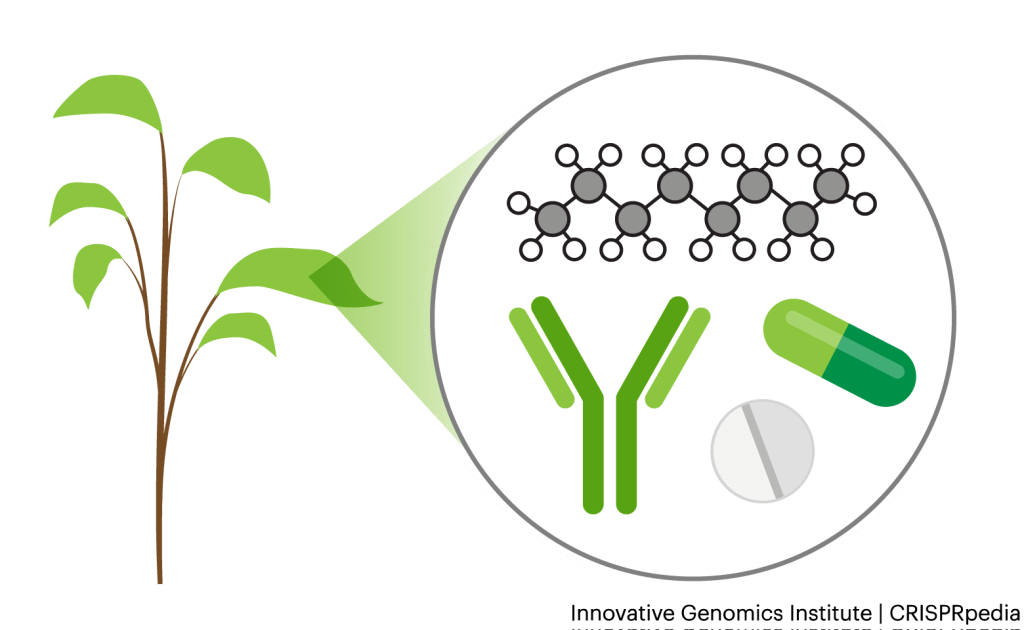
Researchers and biotechnology companies make therapeutics like antibodies, insulin, and vaccines, as well as other organic compounds using cells. They often use bacteria and yeast cells for these processes, but for certain products, researchers use plants instead. For example, the experimental Ebola vaccine, ZMapp, was produced using tobacco plants.
CRISPR tools can make it much easier to turn plants into therapeutic factories. Using these tools, scientists can quickly design and insert therapeutic-encoding DNA into plants, resulting in effective and efficient therapeutic production.
Researchers can also use plants to create industrial chemicals. For example, they can use CRISPR tools to get plants to make more of their natural oils. They can also alter these oils to make them more useful to humans, including ingredients used in fuels, lubricants, flavors and fragrances. It is also possible to alter the sugar content of plants, making the altered plants easier to ferment into products like ethanol.
GENETIC CONTAINMENT
Some people worry that plants with engineered genetic changes will spread their altered DNA to wild species. This could happen if the altered plants are able to breed with nearby wild plants. Crop plants are often too genetically different from their wild counterparts to be able to breed with them. Many conventional plant breeding practices, including the common use of hybrid crops that are sterile, also prevent crop plants from breeding with nearby wild relatives. This is known as genetic containment. Genetic containment methods can be used alongside physical containment methods that put distance or physical structures like walls between altered plants and their wild counterparts.
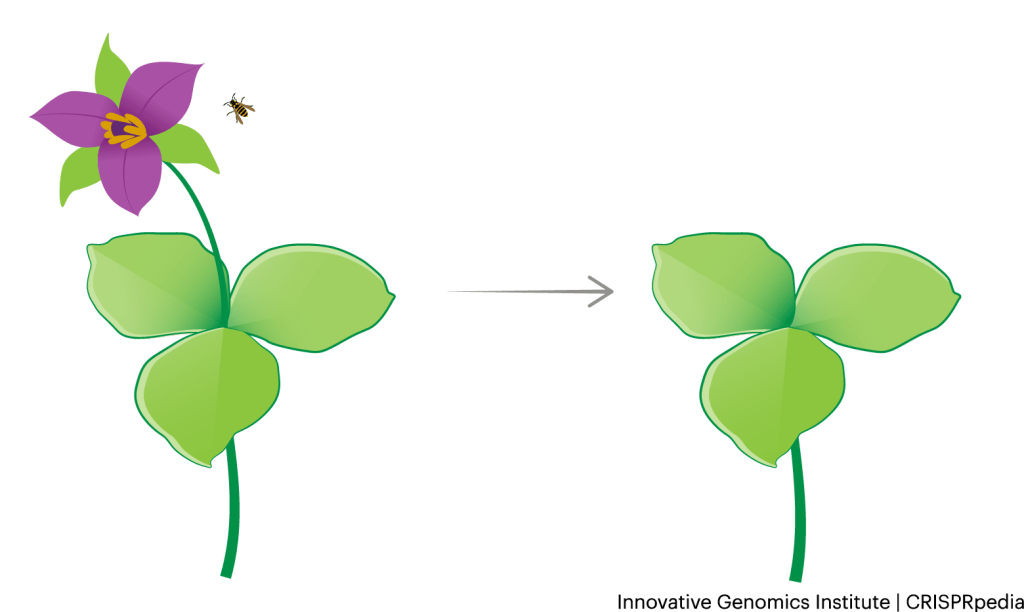
CRISPR tools could be used to create new methods of genetic containment for genetically altered crops. For example, researchers can create plants that don’t produce viable flowers or pollen. For angiosperms, flowers contain plants’ reproductive organs, and without them, they can’t reproduce sexually. For example, organic farmers in Yolo county, California, grow sunflowers with sterile pollen so they don’t cross-pollinate their nearby sunflowers that are grown for seed. Alternatively, farmers can propagate many crops vegetatively. In vegetative propagation, farmers cut off parts of plants that can be regrown into full plants. This allows the plants to be propagated without accidental cross-breeding with neighboring plant varieties or wild species.
EDITING FOR CLIMATE CHANGE AND CONSERVATION
Climate change is the single biggest challenge facing humanity today, and researchers are looking to all possible tools to help society adapt and reverse the effects, including CRISPR genome editing. Researchers are using CRISPR tools to address climate change in three core ways:
- Helping crops adapt to changing climate conditions – Researchers are using CRISPR tools to develop crops that can withstand drought, increased temperatures, and increased salinity.
- Reducing greenhouse gas emissions from agriculture. Microbes are the largest source of greenhouse gas emissions from agriculture. Researchers are using CRISPR tools to study these microbe communities, or ‘microbiomes.’ Learn more here:
- Using CRISPR-based genome engineering to enhance the natural ability of plants and soils to capture carbon from the atmosphere and store it for long periods of time. Increasing plant biomass not only helps make agriculture more efficient, it reduces the need for chemical fertilizers and captures more carbon from the atmosphere.
When it comes to conservation, various wild plant species are threatened by pests and infections, and these threats are increasing due to climate change.

For example, in the Western United States, climate change is increasing bark beetle populations in conifers, which are, in turn, making wildfire problems worse. On the East Coast of the U.S., a fungal pathogen that causes chestnut blight killed over 4 billion American chestnut trees in the 20th century, devastating wild ecosystems and affecting communities that depended on them. CRISPR tools could potentially be used to make these species pest- or infection-resistant, helping to restore biodiversity and aid in plant conservation.
🎥 | Learn more about the American chestnut and different approaches to restoring it: crossbreeding, genetic engineering, and CRISPR-based genome editing.
The potential of CRISPR tools comes with possible ecological impacts and ethical questions. Researchers and affected communities must consider these impacts before releasing modified species into a wild setting. The concerns are similar to those described in-depth for gene drives in the CRISPR Technology chapter of this resource and include that modified plants could change entire species; impact on humans that live alongside these species and use them for food, practical, or spiritual purposes; and unpredictable ripple effects across a whole ecosystem. Any decisions to put modified species into natural ecosystems must be made jointly with affected communities.
FUTURE APPLICATIONS OF CRISPR TOOLS IN PLANTS
We are only at the start of CRISPR-based efforts to breed plants that can fight pests, feed more people, produce therapeutic compounds, and more. We’ll likely see impacts from these efforts soon. Ever more creative applications of CRISPR tools will appear in the years to come.
Engineering farm animals
The many methods to produce livestock with desired traits
VOCABULARY
Selective breeding, conventional breeding, embryo, mosaicism, mosaic, somatic cell nuclear transfer/cloning, clones, somatic cells, nucleus/nuclei, pluripotent
Like plants, animal modification efforts — to get farm animals that were domesticated, easier to herd, and produced more milk or meat — began thousands of years before people knew what DNA was. In this section, you’ll learn about different ways to alter animal DNA, from ancient to modern, and how CRISPR genome-editing could revolutionize animal modification for agriculture.
CONVENTIONAL LIVESTOCK BREEDING
The cows, pigs, chickens, sheep, and goats we keep as livestock today are quite different from their wild ancestors. Farmers keep separate breeds of livestock for specific purposes. For example, egg-laying chickens (“layers”) produce more eggs than meat-producing chickens (“broilers”), while meat-producing chickens have more muscle mass for consumption.
Farmers, and later industrial agriculture companies, achieved this level of livestock specialization with selective breeding. This happens by a similar process in livestock as in crop plants: they wait for desired traits to occur naturally in their animals. Then they selectively mate animals with the trait. After many generations of matings, farmers get breeds with good mixes of traits for specific purposes. This is also known as conventional breeding.
While selective breeding has been going in different forms for millennia, industrial animal agriculture is one of the biggest industries in the United States. Purebred cattle, for example, are part of a highly specialized and tracked industry. There are registries of all the sperm-producing bulls along with their genetic information and unique qualities. Commercial farmers order sperm from these breeders and artificial inseminate cows, tracking economic performance of offspring. There are similar industrial systems for other livestock as well.
Modern conventional breeding uses genetic information and occasionally older genome-editing techniques. CRISPR is just starting to be part of the toolkit. By using CRISPR tools, agricultural companies are starting to quickly generate livestock breeds with desired or new traits. Read on to learn about different ways researchers are incorporating CRISPR tools into animal agriculture.
EMBRYONIC GENOME EDITING
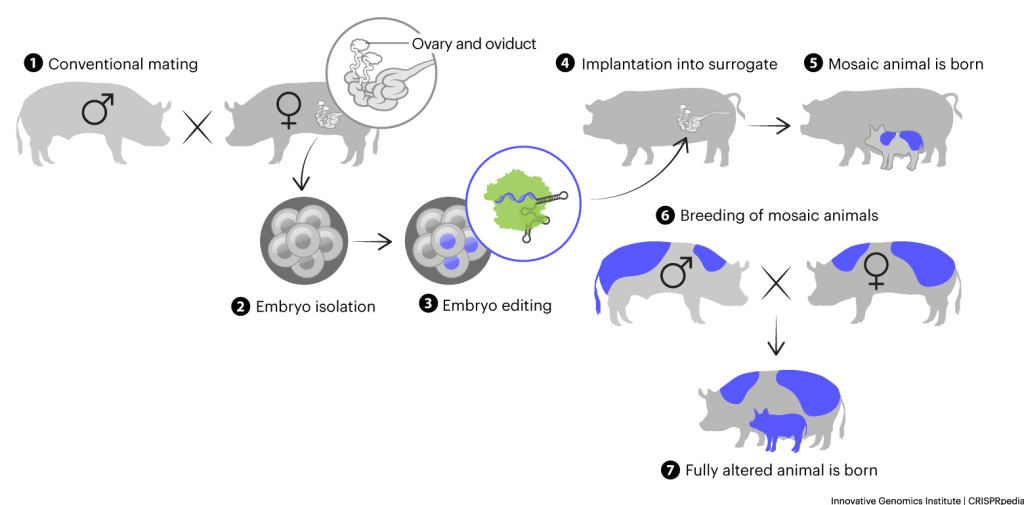
Researchers would like to change livestock traits by altering animal DNA. When a fertilized egg begins to divide, scientists call the resulting mass of cells an embryo. Researchers can isolate embryos and alter their DNA with CRISPR. These embryos can then be implanted into surrogate mothers and grown into full animals with the desired trait. The drawbacks of embryonic editing are mosaicism and inefficient editing:
- Mosaicism – Embryos are made of multiple cells, each with two copies of the genome (usually, one copy from each biological parent) in the nucleus. When CRISPR or other genome-engineering tools are applied to embryos, they are usually able to alter some of the copies of the genome, but not every copy in every cell. So, animals grown from altered embryos don’t usually have 100% altered cells. They have some cells with altered DNA and some with unaltered DNA: this mixture is called genetic mosaicism. Mosaic animals might show the desired trait, but whether or not they can pass it to their offspring depends on whether their sperm or egg cells have the DNA changes. Or they might not show the desired trait, but still be able to pass it down if their sperm or egg cells are altered.
- Inefficient editing – When doing genome engineering research, scientists have many cells to work with. They can try to alter DNA multiple times until they get it right. They can also examine lab grown cells to find the rare ones with the desired changes. When editing embryos, scientists only have a few embryos to work with and it is difficult to alter enough of them to produce the correct DNA changes. As techniques improve and editing becomes more efficient, more DNA changes will be workable.
CLONING
You may have heard of cloning. Cloning has long been of interest in farmers and the animal agriculture industry as a way to reproduce animals that already have desired traits. Getting traits like robust health, high muscle mass, and high fertility through genetics, rather than use of antibiotics, growth hormones, or other drugs, is generally preferred by both breeders and consumers. Through cloning, livestock breeders can create a genetic copy of an existing animal. On a genetic level, this is like creating an identical twin.
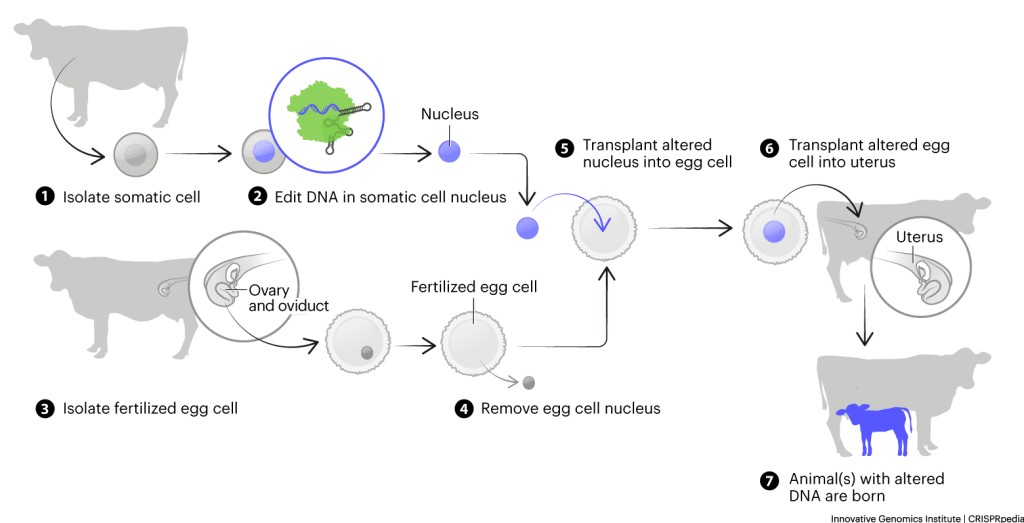
In animal cloning, researchers use adult animal cells called somatic cells to create identical clones. This process makes use of a technique called ‘somatic cell nuclear transfer’ (SCNT). Scientists use SCNT extensively in the creation of genome-edited animals.
Remember that animal cells have subcompartments. These are like tiny “rooms” throughout the cell. One of these rooms is called the nucleus(plural: nuclei) and it contains the cell’s DNA.
Eggs and sperm combine their nuclei during fertilization. As a result, their DNA mixes. Fertilized eggs then grow and divide to form fetuses that eventually grow into baby animals. All cells in these animals contain DNA from their parent eggs and sperm.
SCNT circumvents the fertilization process. Sperm and egg cells do not combine in SCNT. Instead, researchers remove the nucleus from a fertilized egg and replace it with the nucleus of an adult cell.
The egg’s new nucleus acts like its new CEO. Once implanted into a surrogate mother, the egg cell still grows into a full animal. But it does so under the direction of the donated nucleus and its DNA. So, the egg forms an animal genetically identical to the animal that donated the nucleus: a clone. This is the process that created the famous sheep Dolly in the 1990s.
Hello, Dolly!
Dolly, a female sheep born in 1996, was the first mammal cloned by transfer of a nucleus from an adult cell into a fertilized egg. Cloning had been done a few times before, but the donor nucleus had been from other embryonic cells. Embryonic cells are ‘pluripotent’ – they have the ability to turn into any kind of cell in the body. Mature, adult cells, like the ones that make up your bones, liver, or heart, have a defined cellular identity. At the time, most scientists believed it wouldn’t be possible to make a whole organism from the nucleus of an adult cell, but Dolly graced the covers of newspapers and magazines, proving otherwise and opening a new world of possibilities. Learn more about the impact of Dolly the sheep here.
SCNT is very technically challenging and, more often than not, attempts at SCNT are not successful. Sometimes eggs die after implantation. Even if successfully implanted, they often perish during fetal development. And many cloned animals that make it through development only live a short period of time. Researchers continue to refine their techniques to get better success rates with SCNT.
Despite these challenges, it is sometimes possible for researchers to create animals with edited DNA using SCNT. First, they isolate cells from adult animals. Then they grow these cells in the lab. In the lab, they can use CRISPR tools to edit the cells’ DNA. Later, they take the nuclei from these edited cells and use them in SCNT. Any full-grown animals that result with have edited DNA and should display the desired traits encoded in their edited DNA and be able to pass the trait onto their offspring.
SPERM GENOME EDITING
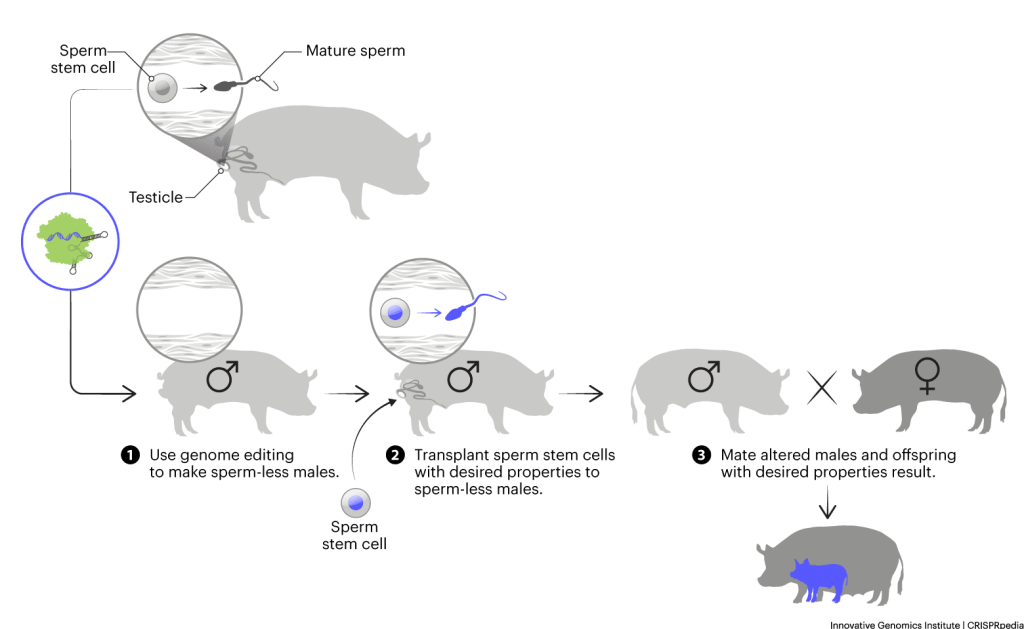
In the strategies described above, scientists alter cells before implanting them into surrogates. Successful implantation has a lot of logistical challenges. In particular, the surrogate mothers must be biologically ready for implantation. Even so, the success rate for implanting a fertilized egg or embryo is much lower than for fertilization that occurs the old-fashioned way — through mating.
To bypass this challenge, instead of altering eggs or embryos, researchers sometimes alter males so that they don’t produce their own sperm. They can then provide the males with sperm stem cells that are altered to produce sperm with the desired trait. These males can then do the legwork of finding receptive females and mating, resulting in their sperm creating altered animals through conventional breeding. Prominent animal biotechnology research Alison Van Eenennaam call this, “artificial insemination on legs.”
Applications of CRISPR in livestock
CRISPR tools could be used in determining offspring sex, increasing muscle mass, improving disease resistance, improving heat tolerance, and more.
CURRENT & FUTURE APPLICATIONS OF GENOME EDITING IN LIVESTOCK
Using CRISPR enzymes to make targeted DNA edits, livestock with a desired trait can be made in as little as a single generation. Researchers hope to make livestock breeds with a number of new traits. Many applications mentioned are aimed at making industrial agriculture more efficient and increase yields and profit, while others are aimed at traits that consumers value.
OFFSPRING SEX
- Cattle that produce offspring of only one sex:
- As with chickens, specific breeds of cattle produce high quality meat. Within these breeds, males produce better meat than females. So, some farmers would prefer to get only male offspring to increase the amount of high quality meat they produce. Researchers are using CRISPR tools to insert a DNA sequence into some bulls of this breed so that they only have male offspring. All these males should produce high-quality meat.
- In India, there are laws preventing the slaughter of cattle, which are sacred in the Hindu religion practiced by most of the country’s residents, but milk cows are still valuable. People who have bulls often turn them out into the streets because feeding and housing them is a burden. Genome editing to produce only female offspring has been proposed as a potential solution.
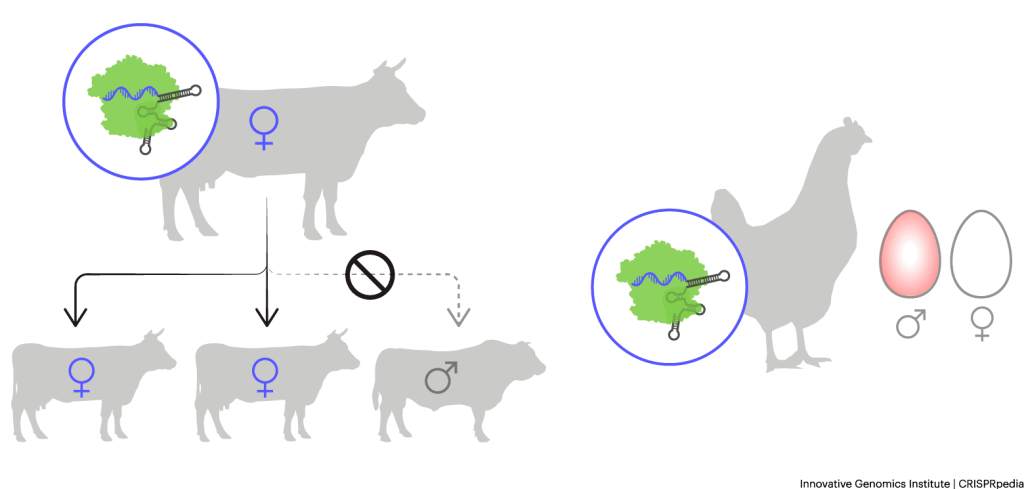
- Chickens with eggs that glow red if they contain males: Females from egg-laying chicken breeds are more valuable than males. In fact, farmers often euthanize males from these breeds. If farmers could identify male eggs before they hatch, farmers could preferentially keep female eggs for breeding and sell male eggs for eating. Researchers in Australia are using CRISPR tools to make male chicken eggs glow red under a special kind of light to allow for fast sorting.
INCREASING MUSCLE MASS

An animal’s muscle mass partially determines the quality of the meat it produces. Some farmers would like to increase their livestock’s muscle mass to produce more and high quality meat using fewer resources.
Researchers know of a particular DNA sequence that puts the brakes on muscle production. They plan to use CRISPR tools to remove or disable this DNA sequence. Animals with this alteration should grow more muscle as a result. Indeed, researchers have already used CRISPR tools to do this in pigs, rabbits, and goats. In some cases there have been tradeoffs: the resulting animals have increased muscle mass, but also have health problems. Other cases have been more successful: in 2022, Japan approved the sale of a gene-edited sea bream that produces 20% more meat following the knockout of a gene using CRISPR. Research in this area is ongoing.
IMPROVING DISEASE RESISTANCE
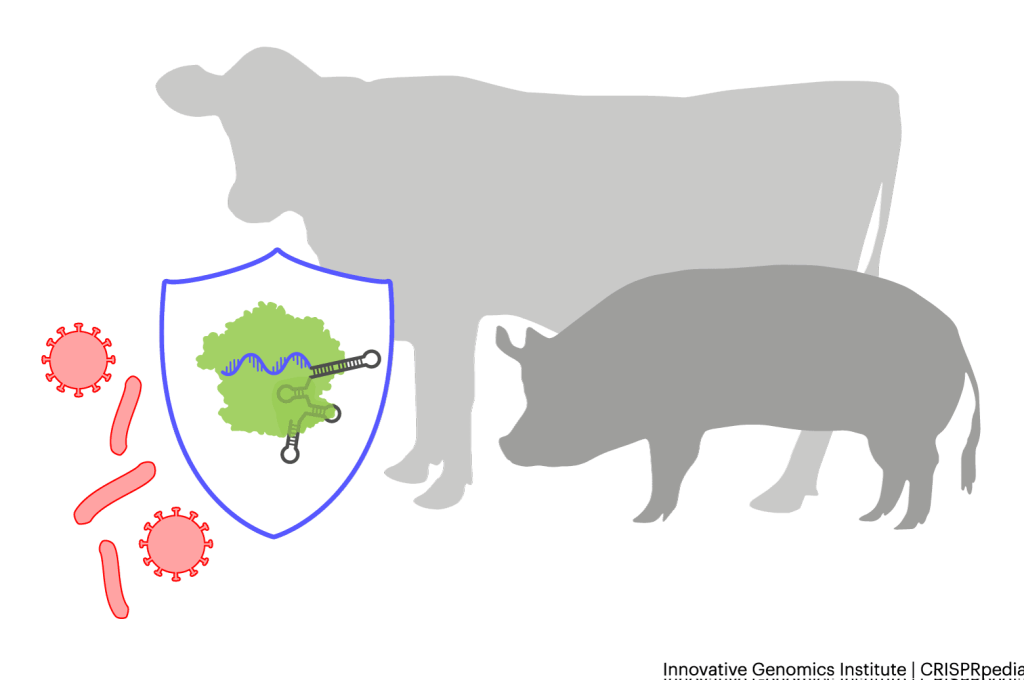
Livestock sometimes get infections. A specific pathogen, like a certain bacteria or virus, will use a specific protein on the outside of the cell, kind of like its very own door handle. Researchers are using CRISPR tools to delete the DNA sequences encoding some of these “handles” in livestock, giving them resistance to certain infectious diseases. Tuberculosis in cattle and PRRSv in pigs, both respiratory illnesses which can spread rapidly when animals are kept in close quarters, are two of the current targets.
IMPROVING HEAT TOLERANCE

High temperatures can be dangerous for livestock. In cattle, high temperatures can reduce milk yield and even lead to heat death. “Slick” cattle — who have shorter, shinier hair — are a naturally found variant that live in the Caribbean. Their unusual coat helps them stay cooler at higher temperatures. Researchers have identified the gene variant responsible for this trait, and have used CRISPR-Cas9 editing to replicate the trait in American beef cattle that may reach consumers in the next few years.
Edited slick beef cattle may be used to adapt cattle ranching to a rapidly warming planet, or to establish cattle populations in hot climates where cattle are not native.
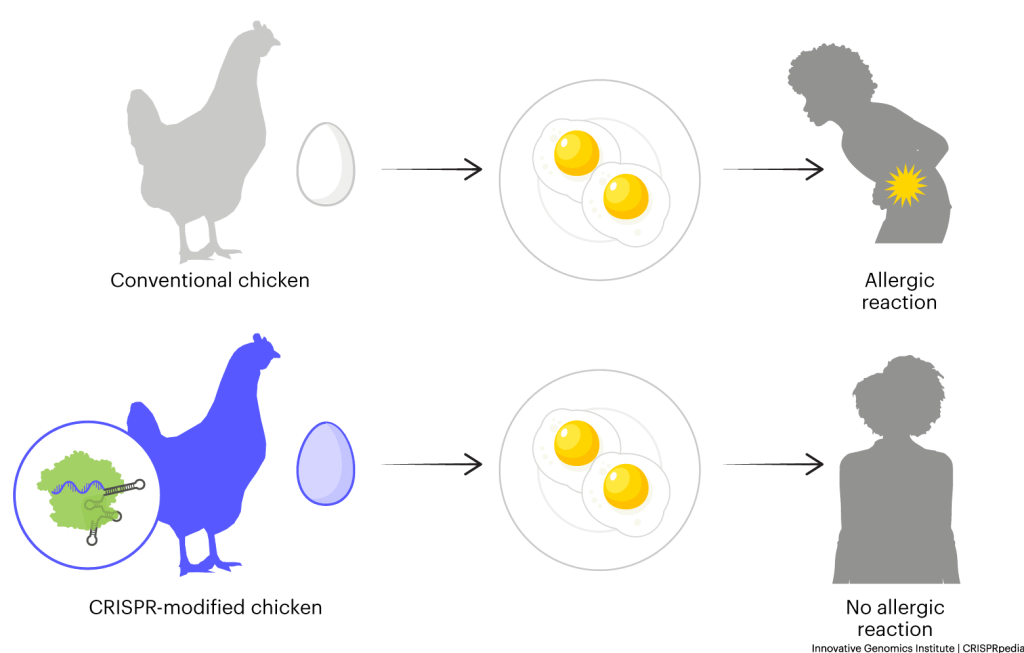
REMOVING ALLERGENS
Researchers know some of the cellular proteins that make people allergic to animal products. They hope to use CRISPR tools to delete or disable the DNA encoding these proteins. Products produced from animals altered in this way should be less allergenic.
ETHICAL CONCERNS ABOUT GENOME EDITING IN LIVESTOCK
Some people are wary of genome editing livestock. From an animal welfare perspective, there are concerns that genome editing will be used to further enable troubling industrial agriculture practices. Crowding is a good example: giving animals adequate space to roam and keeping them in clean quarters is an effective way to prevent the spread of respiratory diseases, but economic incentives drive industrial agriculture to crowd animals. Editing animals so that they can avoid spreading common diseases may lead to them being packed in tighter, more unsanitary conditions that maximize profit over animal welfare. Other people have ethical concerns about whether editing animals and humans is “playing God,” and from the fact that genome engineering techniques perfected in other mammals could ultimately be used in humans. And still other ethical concerns center around environmental harm and the already large climate impact of animal agriculture. These concerns must be discussed when considering whether or not CRISPR should be used to edit livestock.
THE FUTURE OF GENETICALLY MODIFIED LIVESTOCK
This is just a smattering of the many ways we can get new traits in livestock using CRISPR genome editing. Another area of active research is using genome editing to reduce the impact of livestock on climate change by altering the microbial communities that create methane in animal guts.
It has historically been difficult to get genetically-modified animals approved for human consumption. This is partially due to the fact that older genetically-modified animals were often transgenic, with inserted DNA sequences from other species, and are automatically put into a special category of regulation in some countries. In contrast, many of the DNA changes made with CRISPR tools could come from conventional breeding, it would just take much longer. At this point, regulations on CRISPR-edited animals in the US are stringent as well. For now, China is leading the way in animal agriculture applications.
We encourage you to use and adapt CRISPRpedia illustrations for non-commercial, educational purposes. Tweet us @igisci to show us how you’re using them! ALL uses and modifications must follow the Creative Commons BY-NC-SA 4.0 International License.
SCIENTIFIC REVIEWER
Pam Ronald is a Distinguished Professor in the Department of Plant Pathology and the Genome Center at the University of California, Davis. The Ronald lab studies genes that control resistance to disease and tolerance of environmental stress with the goal of improving food security for the world’s poorest farmers. Pam also directs the UC Davis Institute for Food and Agricultural Literacy which cultivates a community of researchers dedicated to making scientific research accessible, relevant, and interesting to everyone.
SCIENTIFIC REVIEWER
Melinda Kliegman holds a Ph.D. in Biology from Stanford University. Before joining the IGI, she worked at the Bill & Melinda Gates Foundation, where she was a Fellow in Global Policy and Advocacy, advising on the use of genome editing in agriculture and human health. In an earlier role at the U.S. Department of Agriculture, Melinda served as a Science Advisor to the Foreign Agricultural Service, where she helped design and fund international trade programs and worked with the UN Convention on Biological Diversity and the UN Food and Agriculture Organization on international policy around plant biotechnology.
HOW TO CITE
Ronald, P.C. & Kliegman M. (2022) CRISPR in Agriculture. In M.L. Hochstrasser et al. (Eds.) CRISPRpedia. Berkeley: Innovative Genomics Institute, University of California, Berkeley. Retrieved from: https://innovativegenomics.org/crisprpedia/crispr-in-agriculture/ (Last updated: September 12, 2022.)
SHARE PAGE