CRISPR genome editing of the staple crop cassava to eliminate toxic cyanogen production
Project Overview
We are using CRISPR to engineer cassava with reduced levels of the poison cyanide.
Cassava (Manihot esculenta Crantz), also known as yuca, manioc, and tapioca, is a staple food for over 800 million people worldwide. This starchy root crop is a major source of calories for roughly 40% of Africans, and an excellent food security crop due to its tolerance for drought and marginal soils. A major challenge, however, is the presence of toxic cyanogenic glucosides in cassava, which must be removed by time-consuming post-harvest processing. Excessive consumption of the released cyanide, in combination with protein-poor diets, can have a number of effects ranging from subtle cognitive problems to konzo, a disease characterized by sudden and irreversible paralysis of the legs. This severe disease of poverty has affected hundreds of thousands of people.
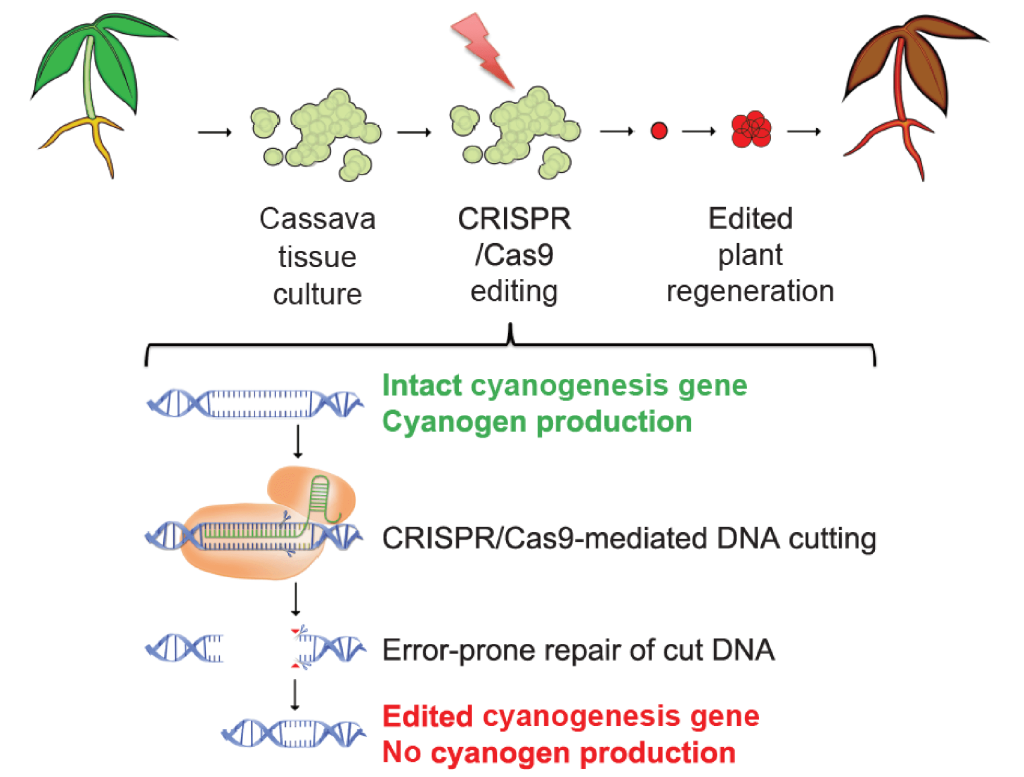
We are working to reduce the levels of toxic cyanogenic glucosides in cassava using CRISPR-Cas9 editing. We are applying Agrobacterium-mediated delivery, which has already been demonstrated in specific cassava cultivars. In parallel, we are developing and applying transgene-free CRISPR-Cas9 delivery methods, which would obviate drawn-out regulatory hurdles. Importantly, smallholder farmers grow various cassava cultivars depending on desired characteristics, such as taste and yield. While it is impractical to apply the slow methods of conventional breeding to such a diverse set of cultivars, genome editing can in principle be applied directly to any cassava cultivar—without disturbing other preferred traits.
We are knocking out, singly and in combination, the genes CYP79D1 and CYP79D2, which catalyze the synthesis of the principal cyanogens in cassava. Knockouts of these genes allow us to address outstanding questions, including the mechanism by which drought-stressed plants upregulate cyanogen production in leaves and roots, and the relationship between cyanogens and protein synthesis. We are assessing the impact of the CYP79D knockouts by examining cyanogen levels and the growth of edited plants, including their tuberous roots. We will also analyze the transcriptomes of edited plants with RNAseq to detect perturbations of gene regulatory networks.
The application of transgene-free CRISPR-Cas9 editing methods to cassava will be a milestone in the broad application of precision breeding to farmer preferred cultivars. We aim to (1) develop these methods, and (2) show that they can be used to modify and investigate a critical trait, cyanogenic glucoside production, in this important food security crop.
Learn more:
- Article: Using CRISPR Genome Editing to Make Cyanide-Free Cassava
- Jess Lyons and Michael Gomez on the CRISPR Cuts Podcast
- Or watch the video below
Principal Investigators
- Jessica Lyons
- Myeong-Je Cho
- Daniel Rokhsar
- Brian Staskawicz
Researchers
- Baljeet Gill
- Michael Gomez
- Nicholas Karavolias
Publications
CRISPR-Cas9-mediated knockout of CYP79D1 and CYP79D2 in cassava attenuates toxic cyanogen production
Gomez MA, Berkoff KC, Gill BK, Iavarone AT, Lieberman SE, Ma JM, Schultink A, Karavolias NG, Wyman SK, Chauhan RD, Taylor NJ, Staskawicz BJ, Cho MJ, Rokhsar DS, and Lyons BJ. Frontiers in Plant Science
Current status and impending progress for cassava structural genomics.
Lyons JB, Bredeson JV, Mansfeld BN, Bauchet GJ, Berry J, Boyher A, Mueller LA, Rokhsar DS, and Bart RS. Plant Molecular Biology
Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence.
Gomez MA, Lin DZ, Moll T, Chauhan RD, Hayden L, Renninger K, Beyene G, Taylor NJ, Carrington J, Staskawicz B, and Bart R. Plant Biotechnology Journal
