NguyenLab

The NguyenLab, led by PI David Nguyen, is located within the Center for Translational Genomics at the Innovative Genomics Institute on the UC Berkeley Campus.
Our research interests lie at the intersection of human immunology, infectious diseases, human genome engineering, and gene therapy. Our goal is to leverage the latest technologies in gene editing to understand how mutations cause human diseases of the immune system and to advance gene correction therapies to treat patients with a Primary Immunodeficiency (PID).
Background
Each year thousands of patients develop life-threatening infections, crippling autoimmunity, or other manifestations of an inborn error of immunity that are poorly understood and difficult to treat. Early PID diagnosis is paramount for prognosis, counseling, and treatment success. Sadly, a causative mutation remains elusive for many patients despite genome sequencing because of an inability to distinguish the truly damaging mutations from the numerous (often benign) genetic sequence variants of unknown significance (VUS). While mutations in over 430 different genes have been shown to cause some form of PID disease, each individual patient’s specific mutation is rare, unlikely to be previously investigated, and thus difficult to identify without further functional lab-based studies.
Instead of relying upon genetically dissimilar animal or cancerous cell lines, the NguyenLab leverages gene editing in primary human immune cells to create more pertinent models of disease. Recreating PID patient’s VUS mutations directly in primary human immune cells provides valuable insight into the genetic underpinnings of the human immune response, could uncover novel mechanisms of immune function, and helps us to ultimately develop curative cell and gene therapies.
Approach
The NguyenLab is founded upon continuous improvements in genome engineering technologies in primary human cells. As we build better gene-editing platforms, we are addressing unmet needs for helping patients with PID diseases:
- Genome engineering in primary human cells to recreate, identify, and diagnose patient-specific PID mutations
- Functional CRISPR screens to understand and predict how PID mutations impact immune cell behavior
- Correcting pathogenic mutations in the immune and blood (hematopoietic) system – including PIDs and hemoglobinopathies
We are currently investigating three disease areas:
- Severe combined immunodeficiency (SCID) and immune regulatory disorders (PIRDs) due to mutations in the interleukin receptor – JAK – STAT signal transduction pathways
- Mutations causing familial hemophagocytic lymphohistiocytosis (HLH) syndromes.
- Mutations pertinent to correcting hemoglobinopathies particularly sickle cell disease
Current Projects
- In order to diagnose and treat a PID disease, it is ideal to understand with certainty that a patient’s mutations are disease-causing. This is particularly critical for developing a gene edited curative therapy.
- We are leveraging CRISPR gene editing to fulfill a ‘genetics Koch’s postulates’. We recreate VUS in primary human immune cells, then test how these mutations alter immune cell function.
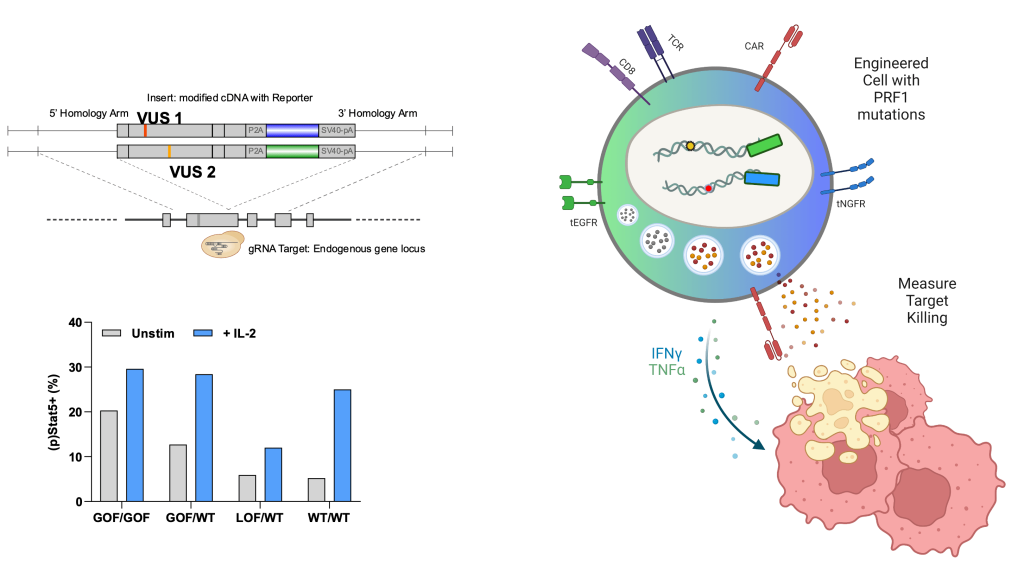
- As genome sequencing is becomes cheaper and more accessible, it is emerging as a frontline test for patients with suspected PID but also uncovering highly suspicious VUS at a pace unmatched by laboratory studies. Waiting to study each patient’s mutation as they present to medical care is impractical for achieving a rapid diagnosis with certainty.
- The NguyenLab employs a pooled variant CRISPR knock-in screening approach (somewhat akin to saturation mutagenesis) using large libraries of single nucleotide variants overwriting a targeted portion of the most common PID genes. We can thus catalog potential disease-causing mutations a priori before they are found in a patient. This approach also builds more detailed fundamental knowledge of human genetic control of the immune system.
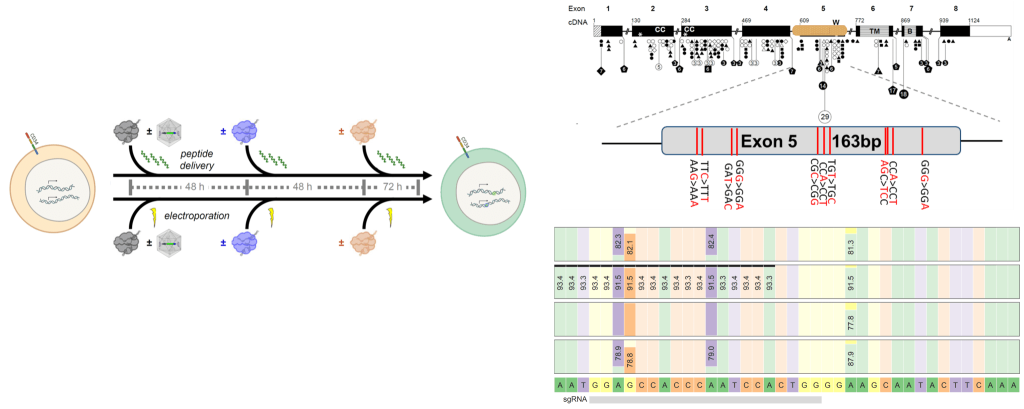
- Virus-based gene insertion therapy strategies are difficult to reconfigure, limited in disease scope, and raise safety concerns due to semi-random genome integration without natural regulation of gene expression. Safer, cheaper gene editing for mutation correction in primary human cells (T, NK, B, HSCs) could vastly expand the number of patients who can access curative gene therapy globally.
- The NguyenLab collaborates with labs around the IGI and beyond to develop and apply novel tools for improved delivery of CRISPR gene editing reagents. We are studying peptide-mediated editing and molecular strategies for ‘gene patches’ to overwrite patient mutations with healthy code. Further, we are part of IGI-wide efforts to assess how various mutation correction strategies impact the diversity of cellular functions post-editing.
Recent Publications
Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes
Foss DV, Muldoon JJ, Nguyen DN, Carr D, Sahu SU, Hunsinger JM, Wyman SK, Krishnappa N, Mendonsa R, Schanzer EV, Shy BR, Vykunta VS, Allain C, Li Z, Marson A, Eyquem J, and Wilson RC. Nature Biomedical Engineering
High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails
Shy BJ, Vykunta VS, Ha A, Talbot A, Roth TL, Nguyen DN, Pfeifer WG, Chen YY, Blaeschke F, Shifrut E, Vedova S, Mamedov MR, Chung JJ, Li H, Yu R, Wu D, Wolf J, Martin TG, Ye L, Esensten JH, Eyquem J, and Marson A. Nature Biotechnology
Constrained chromatin accessibility in PU.1-mutated agammaglobulinemia patients
Le Coz C, Nguyen DN, Su C…Marson A, Poon GMK, and Romberg N. Journal of Experimental Medicine
Targeted delivery of CRISPR-Cas9 and transgenes enables complex immune cell engineering
Hamilton JR, Tsuchida CA, Nguyen DN, Shy BR, McGarrigle ER, Espinoza CRS, Carr D, Blaeschke F, Marson A, and Doudna JA. Cell Reports
Functional CRISPR dissection of gene networks controlling human regulatory T cell identity.
Schumann K, Raju SS, Lauber M, Kolb S, Shifrut E, Cortez JT, Skartsis N, Nguyen VQ, Woo JM, Roth TL, Yu R, Nguyen MLT, Simeonov DR, Nguyen DN, Targ S, Gate RE, Tang Q, Bluestone JA, Spitzer MH, Ye CJ, and Marson A. Nature Immunology
Timed inhibition of CDC7 increases CRISPR-Cas9 mediated templated repair.
Wienert B, Nguyen DN, Guenther A, Feng SJ, Locke MN, Wyman SK, Shin J, Kazane KR, Gregory GL, Carter MAM, Wright F, Conklin BR, Marson A, Richardson CD, and Corn JE. Nature Communications
Pooled knock-in targeting for genome engineering of cellular immunotherapies
Roth TL, Li PJ, Blaeschke F, Nies JF, Apathy R, Mowery C, Yu R, Nguyen MLT, Lee Y, Truong A, Hiatt J, Wu D, Nguyen DN, Goodman D, Bluestone JA, Ye CJ, Roybal K, Shifrut E, and Marson A. Cell
Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency.
Nguyen DN, Roth TL, Li PJ, Chen PA, Apathy R, Mamedov MR, Vo LT, Tobin VR, Goodman D, Shifrut E, Bluestone JA, Puck JM, Szoka FC, and Marson A. Nature Biotechnology
Reprogramming human T cell function and specificity with non-viral genome targeting.
Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, Hiatt J, Saco J, Krystofinski P, Li H, Tobin V, Nguyen DN, Lee MR, Putnam AL, Ferris AL, Chen JW, Schickel JN, Pellerin L, Carmody D, Alkorta-Aranburu G, del Gaudio D, Matsumoto H, Morell M, Mao Y, Cho M, Quadros RM, Gurumurthy CB, Smith B, Haugwitz M, Hughes SH, Weissman JS, Schumann K, Esensten JH, May AP, Ashworth A, Kupfer GM, Greeley SAW, Bacchetta R, Meffre E, Roncarolo MG, Romberg N, Herold KC, Ribas A, Leonetti MD, and Marson A. Nature
See the full list of PI David Nguyen’s publications on Google Scholar
People


Ekansh Agrawal
Undergraduate Researcher

Yaw Ansong-Ansongton, M.D.
Graduate Student

Dror Assa, Ph.D.
Postdoc

Allison Bien
Undergraduate Researcher

Mikayla Bordador
Undergraduate Researcher

Joy Chen
Graduate Student

Utku Goreke, Ph.D.
Postdoc

Dipti Kamath, M.D.
Clinical Fellow


Nathan Perez
Undergraduate Researcher


Mikhail Vysotskiy, Ph.D.
Postdoc
Lab Alumni

Maher Budron
Graduate Student (Rotation)

Brenna Gittins
Graduate Student (Rotation)

Elise Wolf, Ph.D.
Postdoc
News
Recruiting
- We are actively seeking candidates for post-doctoral scientists, research assistants, and graduate students. If you are excited by our work and interested in joining the group, please reach out to David (PI): david[dot]n[dot]nguyen[at]berkeley.edu
Commitment to DEI
- The NguyenLab is eager to engage, support, and train the next generation of scientists and clinicians. All are welcome in our lab — regardless of race, ethnicity, gender identity, sexual orientation, socioeconomic background, or disability. We believe that diversity is a critical component of the educational experience, for maximizing innovative and translational science, and improving patient care and outcomes.
- We foster training programs and personal relationships to promote inclusion of all. We strive to ensure equity and access across all backgrounds and identities, and we are committed to recruiting, mentoring, and training people historically underrepresented in academia and the STEM fields.