Half a Million COVID Tests Later, IGI Clinical Laboratory Looks to the Future
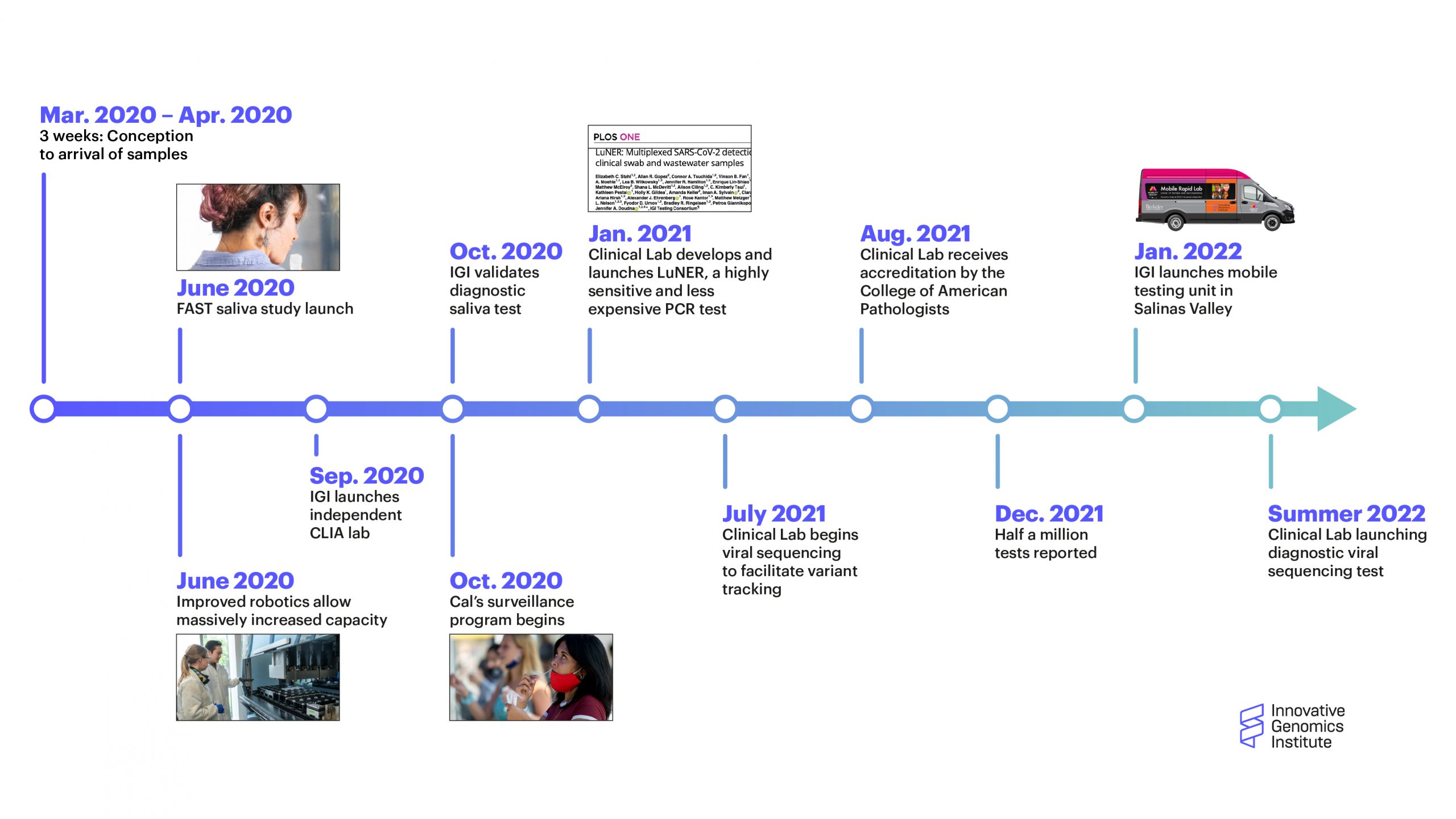
The IGI Clinical Laboratory just passed two major milestones: the two year anniversary of the lab launching in response to the COVID-19 pandemic, and the processing of half a million tests in that time. In early 2020, neither of these milestones could have been foreseen, but now the IGI Clinical Laboratory has evolved into a key part of the IGI and its future vision even after the COVID pandemic is in the rearview mirror.
The Twisty Road to 500,000 Tests
From the first COVID test, the IGI Clinical Laboratory had to constantly innovate and react to a rapidly changing situation. In addition to developing a new testing protocol that lowered costs and improved the sensitivity of the test, the lab created and validated a new saliva test across the UC campus, developed robotic workflows and sample pooling for handling the volume of testing needed by the University and community partners doing testing for underserved communities and first responders around the Bay Area and northern California, and coordinated all of this work with public health agencies.
Because of the fast-moving emergency, the IGI Clinical Laboratory was a bicycle that had to be built — and constantly improved — while it was moving.
“Looking back on it, I think about all of the hard work that so many put into this lab and the innovations made along the way,” says Matt McElroy, Operations Supervisor of the IGI Clinical Laboratory. “But not all of the process improvements were exciting in a traditional sense – as an example, we turned on a duplicate workflow to double our testing capacity. Likewise, we trimmed all the fat from our workflows and trained a core crew of testing personnel to test efficiently.”
In January 2022, the lab got slammed by the rapid surge of the Omicron variant. From getting 50–100 positives per week, all of a sudden the lab was getting 1000.
“When the positivity skyrocketed, it stressed every little thing that we do. Things like high positivity rate have a huge impact when performing sample pooling. Samples in positive pools have to be retested, which requires consolidating retest samples from racks. This went from taking a few minutes to hours. So we had to design new ways of finding samples quickly.”

The waves have also impacted the community partners and testing teams that are out in the community and in the UC Berkeley testing centers collecting samples. As positivity rates go up, so does the number of people seeking a rapid turnaround COVID test.
“One of the biggest challenges in the past year has been the massive changes in testing demand during surges,” says Renna Khuner-Haber, the COVID Program Manager for LifeLong Medical Care, who has been a community testing partner of the IGI since early in the pandemic. “When a surge hits, we have to rapidly increase our staffing capacity to accommodate for the sudden increase in testing as well as the skyrocketing number of positive cases.”
When case rates are rising rapidly, rapid turnaround time for tests becomes increasingly important. If a patient is positive and doesn’t know for several days, that increases the chance of spreading it to others.
“The reliability of COVID testing and quick turnaround times is essential for it to be a useful tool and prevent community spread,” says Khuner-Haber. “IGI has consistently had quick turnaround time for testing, even in the beginning of each surge.”
A timeline of major milestones for the the IGI Clinical Laboratory (click to enlarge)
As the Virus Evolves, So Does Testing
“Since the start of the pandemic, the needs and the type of information that our partners require have evolved,” explains Petros Giannikopoulos, Director of the IGI Clinical Laboratory. “At the beginning, just knowing if somebody had COVID or not was what was necessary. So we responded and built up a high-capacity platform to determine if somebody has SARS-CoV-2, and we got better and better at doing that. We developed our own version of the PCR assay that enabled us to improve turnaround time and performance. Through the support of the Packard foundation, we’ve been offering our service to high-risk and vulnerable communities in the East Bay.”

The emergence of new variants changed the landscape of the pandemic around the world. It shifted the way people understood their own risk and prompted the IGI Clinical Laboratory to rethink the type of testing it could provide.
“With Delta, it became very clear that the strain that somebody has may determine how sick they get. So we responded and are launching a clinical SARS-CoV-2 sequencing assay that allows us to report which variant of the virus a patient has if they test positive,” says Giannikopoulos “We’re trying to stay ahead of the curve. In the event that there are other strains that pose specific risks, that kind of information may influence medical management.”
As part of a global collaboration to track the evolution and spread of the virus, the IGI has been sequencing positive tests, but with the new clinical assay that the lab is planning to roll out in May 2022, that information can be relayed to the person who tested positive.
The IGI Clinical Laboratory is also supporting researchers at the IGI who are developing rapid point-of-care tests for COVID-19 and other infectious diseases using CRISPR not as a genome-editing tool, but as a way to quickly identify specific pathogens.
“Because we have a clinical lab in house that performs gold-standard testing, we have a mechanism to benchmark new tools, including new CRISPR-based assays,” says Giannikopoulos.
Why CRISPR Needs a Clinical Lab
While the IGI Clinical Laboratory was created to fill an emergency need, it became clear that having a CLIA-certified clinical lab in the building had additional benefits, particularly for the development of CRISPR-based cures.
A key challenge for new CRISPR-based therapies is measuring the success of an experimental treatment. These therapies are so new, there are no pre-existing tests. For the UC consortium’s current clinical trial of a CRISPR therapy for sickle cell disease, researchers want to be able to track how well the therapy is working and to watch for any unintended side effects. This is where the IGI Clinical Laboratory comes in.
The process of developing a sequencing assay to identify SARS-CoV-2 variants is serving another purpose: providing a foundation for other clinical-grade sequencing assays for the sickle cell disease and other CRISPR-based therapies in development at IGI.
“Our responsibility for the sickle cell trial is to develop and then ultimately deploy a clinical-grade assay to look at the efficiency of editing the sickle cell mutation in patient stem cells, and also to look for one of the known off-target sites in the stem cells and then in peripheral blood and bone marrow over time,” says Giannikopoulos.
COVID Continues
While much of the media attention on the COVID pandemic has shifted to other stories as the Omicron wave appears to be receding, the IGI Clinical Laboratory is preparing for what comes next, and is even working to expand the network of community partners over the coming months.
“Our team works hard to provide dependable, rapid, high-quality testing for our partners,” says Erica Moehle, Project Manager for the IGI Clinical Laboratory. “For better or worse, our Clinical Laboratory has lived through multiple demanding surges, so we better understand the boom and bust cycle of the testing landscape. While we wish the pandemic were over, we are still making sure we are prepared for whatever the future holds.”
 By
Andy Murdock
By
Andy Murdock