
IGI Scientists Make E. coli That Can Take Carbon Dioxide From the Air — And Use It
New study shows that re-engineered E. coli can absorb and concentrate carbon dioxide from the air, taking a step towards carbon sequestration.
Rising levels of carbon dioxide (CO2) in the atmosphere are causing climate change and environmental destruction. But what if we could draw more CO2 out of the atmosphere using living organisms? Better yet, what if we could use the carbon in CO2 to feed more people or fuel a new, green chemical industry? In a paper published today in eLife, a team from IGI researcher Dave Savage’s lab at UC Berkeley reports a major advance in this direction.
Carbon: the real MVP
All life forms on Earth use carbon. Carbon is an essential building block of the cell and of compounds cells use for fuel. Some life forms, notably plants and microorganisms that use photosynthesis, get all of their carbon from CO2 in the atmosphere. But because CO2 makes up just 0.04% of Earth’s atmosphere, it isn’t easy for organisms that rely on atmospheric CO2 to get the carbon they need. Pumping CO2 into a cellular compartment called a carboxysome is a common way many microorganisms concentrate CO2, giving the cell a readily available pool.
Most other organisms, including humans, get carbon from their food. E. coli — bacteria that live in the guts of humans and other animals and are used extensively in scientific research — usually get carbon by eating simple sugars from the foods we consume. Being able to put carboxysomes, and their ability to capture CO2, into new organisms could have huge implications for the environment, agriculture, chemical engineering, and more.
A carbon roadblock
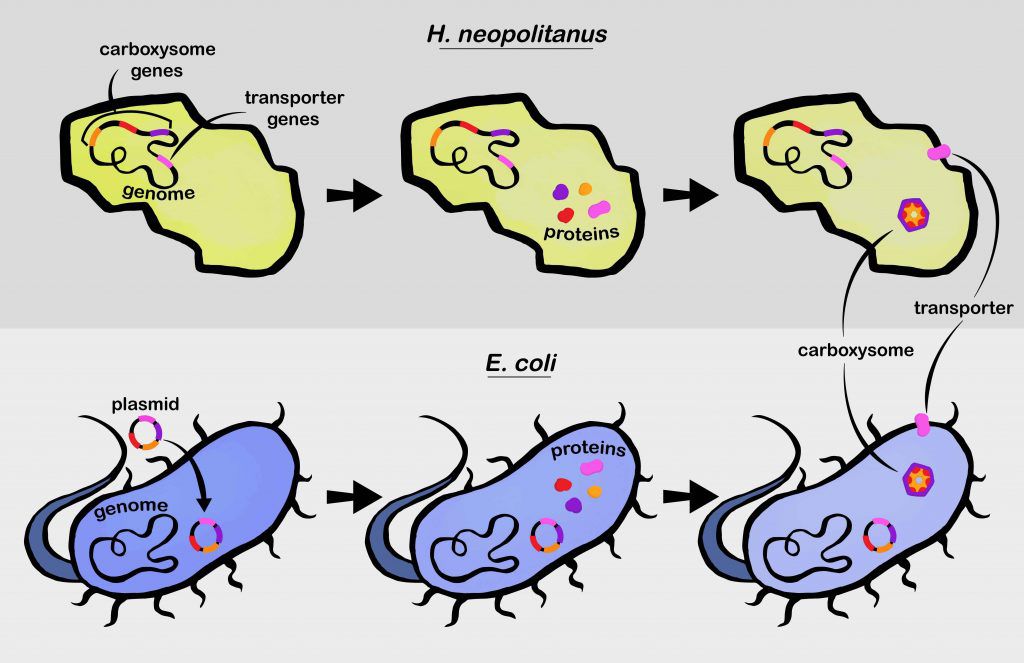
Carboxysomes aren’t simply empty compartments, but complex structures made of more than 10,000 distinct protein pieces that spontaneously self-assemble. In previous work, researchers from the Savage lab identified what they hoped was the full complement of genes needed to make a functioning carboxysome in a microorganism they were studying, as well as crucial transporters, which bring CO2 into the cell. In the work published today, they tested whether they had actually uncovered all of the crucial carboxysome and transporter genes by adding them to E. coli bacteria. Their goal was to recreate a functioning CO2 concentrating system in a foreign host cell that never had one to begin with.
The Savage team was confident they could create something that looked like a carboxysome by inserting the right genes, but they wanted to see if it actually worked like a carboxysome, too. But, normal E. coli get carbon from their diet. They don’t need to concentrate CO2, and will grow happily without it. So, they needed to engineer E. coli that had no option but to use their new carboxysomes and transporters to get the carbon they craved.

“For many years, I’ve been interested in reconstituting the carboxysome to study its structure and remarkable ability to self-assemble. But it wasn’t clear how we could do the home run experiment of functionally reconstituting the entire CO2-concentrating system,” says Savage. “The critical event was Avi Flamholz joining the lab!”
Flamholz, who had previous experience with studying photosynthesis in Ron Milo’s lab at the Weizmann Institute of Science, realized the key was to force the bacteria to look elsewhere.
“We made a roadblock in their metabolism by deleting an important gene,” explains Flamholz. “And then we gave them the opportunity to take a detour around the roadblock by using CO2.”
In other words, the research team removed genes that E. coli usually uses to process sugars. This change meant the E. coli needed another source of carbon — like CO2. But they can only get enough carbon if the transporter and carboxysome work together effectively to concentrate ambient CO2 from the air. It worked.
“Actually productively concentrating CO2 out of ambient air is bananas!” says Savage. “Although atmospheric CO2 levels are rising in a way that is hugely significant for climate, most organisms that use ambient CO2 for their carbon source are practically starving for CO2! To see the system work as hypothesized was very satisfying.”
Feeding a growing planet with CO2
Microorganisms invented CO2-concentrating technology, and this research brings us a step closer to being able to borrow and harness it. There has long been excitement about the possibility of adding engineered CO2 concentrating systems to plants so they can pull more CO2 from the air, potentially slowing climate change.
“We’re very excited about the potential applications, which is one reason it’s so great to be a part of the collaborative research going on at the IGI,” says Savage. “Moving forward we have a large interest in the chloroplast, which is the powerhouse of the plant cell. We think that some of the principles learned here could be used to improve photosynthesis in plants.”
Adding additional CO2-concentrating power to crops could give them more fuel to grow bigger, faster, while reducing water and fertilizer usage. In other words, the aim is to both capture CO2 from the air and create more food to help feed our growing global population with fewer farm inputs.
Greening chemistry out of thin air
In addition to reducing chemical use, this advance could affect how chemicals and chemical reactions are made in the first place. Traditional chemical manufacturing produces heavy metals, petroleum, toxic waste, and releases CO2 into the atmosphere. “Green chemistry” uses engineered organisms to perform chemical reactions, cutting back on these environmental hazards. Transplanting this CO2-concentrating system into E. coli or other microbes used in green chemistry could become one way to actually take CO2 out of the atmosphere, instead of adding more.
The Savage lab and their collaborators are now working on adding carboxysomes and CO2 transporters into industrially relevant microorganisms to see if they can concentrate CO2 and make it into useful products.

“Think about all the things you might possibly make out of microbes, like chemicals, biofuels, additives for food or even bulk edible protein, like imitation meats,” says Flamholz. “For all of those things, why shouldn’t the carbon source be CO2? Why shouldn’t we pull it out of the air?”
You may also be interested in

IGI Seminar Series: Progress and Challenges in Delivering Cassava with RNAi-mediated Resistance to Cassava Brown Streak Disease to Smallholder Farmers in Africa – It’s Not Just About the Technology

IGI Seminar Series: Empowering Teachers, Transforming Classrooms: Advancing K-12 STEM Education

IGI Seminar Series: Writing DNA with RNA: Genome Engineering by Target-Primed Reverse Transcription

 By
Hope Henderson
By
Hope Henderson