New Research Points the Way to CRISPR Therapies for Hereditary Dementias
Using CRISPR in patient cells to get one step closer to the clinic
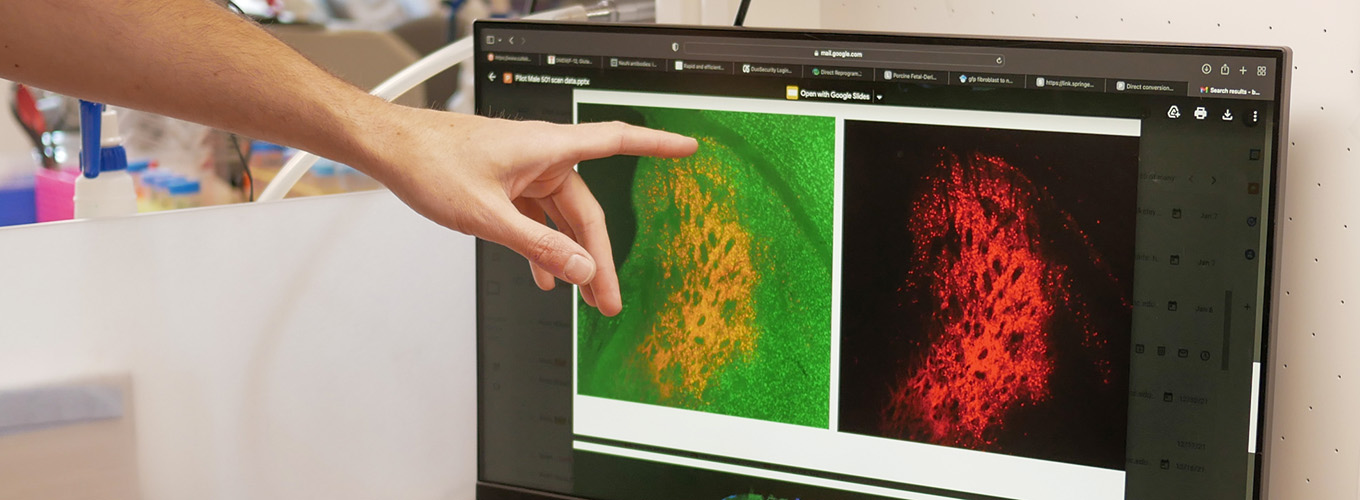
In a new paper in PNAS, IGI physician-scientist Claire Clelland and her team make headway on their goal of creating a treatment for hereditary forms of dementia. Clelland used cells from patients with hereditary ALS and frontotemporal dementia (FTD) to compare different CRISPR-based treatment approaches.

“I became a physician-scientist to try to cure dementia,” says Clelland. “And when I finished my training, I recognized that CRISPR is the best shot for potentially allowing us to cure genetic forms of dementia, and then in the future, more sporadic forms. Our approach in my lab is going after dominant gain-of-function mutations because they are aligned with what CRISPR can do.”
The majority of cases of hereditary ALS and FTD share a common cause: one copy of the C9orf72 gene that has a “repeat expansion” where a short DNA sequence is repeated over and over, like a record skipping and playing the same note on repeat.
The team compared three CRISPR-based approaches, one of which would work to “turn off” the mutated copy of the gene by cutting out a regulatory sequence at the beginning, while the other two actually remove the expansion itself. The first approach was not successful but the second two were.
“It was not clear in the field what genetic change would need to be made,” says Clelland. “So, we compared three editing approaches in patient cells and determined that you need to remove the repeat expansion. You can’t just silence it. So that’s really important information for genetic therapies moving forward, and for understanding why previous treatments attempts have failed.”
Clelland’s work relies on the donation of patient cells from individuals with ALS and FTD. Repeat expansions can’t easily be created in the lab the way smaller disease-causing mutations can. Studying the gene with the native sequences around the mutation is also crucial for understanding the gene function and best way to correct the mutation.
Moving forward, Clelland will continue her work in patient cells as well as animal models. She is working with the IGI Delivery Collective on ways to deliver these novel CRISPR-based therapies for ALS and FTD to the brain, and is part of the NIH-funded CASEPR project at the IGI working toward clinical trials on CRISPR therapies for ALS and Huntington’s Disease.
“I see patients with fatal genetic dementias in clinic. These are multigenerational diseases, where the patient has cared for a parent or sibling with the disease, and by the time they’ve been diagnosed, they may have had children and potentially passed it on to their kids. They’re the most inspiring patients I have ever met. We haven’t had a lot of therapies to offer them so far, but the tide is changing. It’s a really exciting time in neurology because we finally have the tools to create effective therapies.”
Clelland’s work is supported in part by SCGE.
Read more:
 By
Hope Henderson
By
Hope Henderson