For Patients & Families
Overview
Please note: The Innovative Genomics Institute cannot offer any form of medical advice about specific diseases or conditions. We hope that the resources provided below can help guide you to the information you are seeking.
Therapeutic genome editing is still in the early stages of development. Finding a treatment or cure for any disease requires scientific research, extensive safety and efficacy studies, clinical trials, and more. We are years away from turning our current research into an approved treatment option for any disorder. We are committed to seeing this through, but we are not there yet.
In addition to following our updates, you may wish to explore other resources to better understand your or your loved one’s disease, open clinical trials, and approved treatments or get in touch with patient advocacy and support groups for more personal guidance. We have compiled a list of resources to help you get started.
INGENUITI Research Study

The Interventional Genomics Unit for Therapeutic Innovation (INGENUITI) is an initiative at the Innovative Genomics Institute to study the mechanisms underlying how genomic variants can cause disease and how genome-editing tools like CRISPR-Cas9 could be used to treat those diseases.We are actively recruiting participants for this study, which you can read more about here. If you’re interested, you can email ingenuiti@berkeley.edu, and an investigator will get back to you.
What Diseases Might Be Treated with Genome Editing?
The first step in treating a disease is getting a diagnosis — doctors need to know what is causing symptoms before they are able to treat it. And scientists need to understand the biology of how a disease works to know if genome editing might be able to treat it in the future.
Some diseases are genetic, meaning they are caused by differences in DNA that we inherit from our biological parents. These might be DNA variants that “run in the family,” or are spontaneous in the original sperm and egg that came together to form us.
Some genetic diseases, like sickle cell disease and cystic fibrosis, are caused by DNA changes in just one gene. Single-gene diseases are the first targets for genome-editing therapies, simply because editing one gene is easier than editing many. The first of these kinds of therapies are being evaluated in clinical trials for a number of conditions.
Some diseases, like multiple sclerosis and diabetes, are associated with changes in DNA in many genes. The effects of these different variants add up to make someone more likely to get a disease. Editing multiple genes at once is much tougher for scientists than editing just one gene. Genome-editing therapies for these types of diseases are further away.
Not all diseases are caused by DNA changes. Other factors can cause or contribute to disease, too. People who live in areas with more air pollution are more likely to develop asthma — this is an example of an environmental factor contributing to disease. Health habits like diet and exercise contribute to metabolic diseases like type II diabetes. Spontaneous mutations that accumulate in DNA over the course of a lifetime contribute to cancer. Viral and bacterial infections cause yet other diseases, like Hepatitis C. And the causes of many diseases are still unknown. When scientists have a very deep understanding of how a disease works, they might be able to treat it with genome editing, even if it is not a genetic disease. New CRISPR-based cancer therapies, currently being evaluated in clinical trials, are an example of this.
Clinical Trials and the Timeline to Treatment
Basic research is the foundation of modern medicine. The discovery of new CRISPR proteins is an example of basic research. Oftentimes, news headlines suggest that these discoveries will immediately result in new medications or therapies. In fact, basic research is only the first step in a long journey towards new treatments becoming available to patients.
What happens after basic research is complete?
Once scientists have an idea for a new drug or therapy, they will begin “pre-clinical research,” which involves lab experiments to generate appropriate materials, test strategies, and develop a strong formulation. This process takes multiple years and concludes when the researchers submit data and plans for a proposed clinical trial to the U.S. Food and Drug Administration (FDA). Before researchers begin working with patients, they must show that their treatment works safely in human cells outside the body and in lab animals. If the data and plans are sound, the FDA will let the researchers start a human test called a “clinical trial” to make sure the treatment is safe.
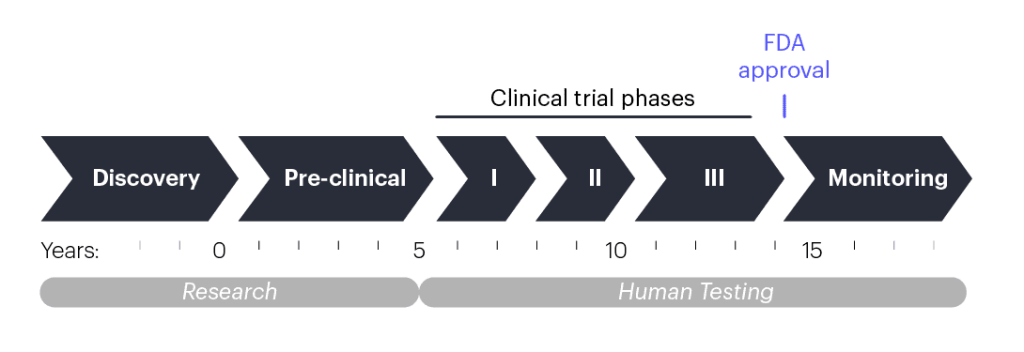
Why does it take so long for new treatments to become widely available?
Clinical trials are conducted in “phases” that each take multiple years. Phase 1 clinical trials typically include just a handful of people, and the goal is just to make sure that the experimental treatment is safe. As phases progress, the number of patients enrolled increases, and in addition to safety, clinicians start measuring whether the treatment improves patient health. After the third or fourth phase, the FDA may approve the treatment for broad public use if it clearly safe and effective. From pre-clinical research to FDA approval, developing a new therapy may take 10–15 years.
Please note that the timeline presented here is a generalized approximation meant to provide an overall sense of the therapeutic development process. Duration of testing phases for any new therapy may vary, and many promising therapies never even make it to approval.
Although such a long timeline may seem daunting, researchers are actively looking for patients to enroll in active clinical trials. In addition to trials using CRISPR-based therapies, there might be other clinical trials for the disease you’re interested in that involve typical drugs, biologics, gene therapy, other gene-editing tools like zinc finger nucleases, and more. You may wish to consider these options as well. To learn more about clinical trials and search through lists of upcoming or active trials, please see the references below.
A note on dubious clinical trials
Unfortunately, a small number of trials are run without proper safety oversight. Your doctor should be able to help you confirm that a trial is legitimate, but here are some things to look for in US-based trials:
- A legitimate clinical trial will have no cost for participation, and may pay patient-volunteers for their time.
- You can withdraw from a legitimate trial at any time with no cost or consequence.
- Legitimate trials are approved by the FDA. The researchers should be able to tell you if a trial is FDA-approved. That said, FDA approval of a clinical trial does not mean that the FDA has approved the therapy being tested: the purpose of a clinical trial is to identify the risks and benefits of a therapy so that the FDA can eventually determine whether the therapy should be approved and broadly available to patients.
- Clinicaltrials.gov is a good source for finding trials, but being listed on that site does not mean a trial is legitimate.
- A legitimate clinical trial will be approved by an Institutional Review Board (IRB). The researchers running the trial will be able to tell you the name of the IRB, and for US-based trials, it will be listed here. You may also be able to search by the name of the institute or company sponsoring the trial.
- A legitimate trial involves informed consent, explained in detail here. Briefly, this includes a researcher explaining possible risks and benefits to potential patient-volunteers before they enroll. A researcher should never try to persuade or pressure you to participate.
To learn more about clinical trials and search through lists of upcoming or active trials, please see the references below.
WHAT ARE CLINICAL TRIALS?
“Molecules to Medicine: Clinical Trials for Beginners” from Scientific American
“Clinical Trials” overview and additional resources from Pharmaceutical Research and Manufacturers of America (PhRMA)
“From Molecules to Medicine: How Are New Drugs and Therapies Developed?” detailed informational guide from rare disease advocacy organization Global Genes
“NIH Clinical Research Trials and You” from the U.S. National Institutes of Health
“Can CRISPR Save Ben Dupree?” perspective piece on the timeline and specific challenges in developing therapeutic CRISPR treatments from MIT Technology Review
HOW DO I FIND ONE?
ClinicalTrials.gov from the U.S. National Institutes of Health
Searchable registry of clinical trials around the world.
Antidote
Online portal that uses relevant demographic information to match patients with any recruiting or ongoing clinical trials around the world.
ASCGT Clinical Trials Finder
Searchable registry of gene and cell therapy trials in the US.
Additional Resources and Personalized Assistance
UNDERSTANDING YOUR OR YOUR LOVED ONE’S DISEASE & CONNECTING WITH SCIENTISTS AND PATIENT ADVOCATES
Centers for Disease Control (CDC)
Provides the public with information and resources on a comprehensive list of diseases and conditions in English or Spanish.
Genetic and Rare Diseases (GARD) Information Center
Gives the public current, reliable, and easy-to-understand information about rare or genetic diseases in English or Spanish.
Genetic Alliance
Empowers individuals, families, and communities with genetic disease information, treatment, and ways to participate in research.
MyGene2
Patient-facing site that offers DNA sequencing to assist in disease diagnosis and clinical understanding of rare disease, provides a platform for patients and families to share their experiences, and connects patients with researchers.
Rare Genomics Institute
Connects patients and their families with both patient advocates and scientific researchers who are studying their specific condition.
Rare Diseases Clinical Research Network and its Coalition of Patient Advocacy Groups
Provides information on rare diseases, clinical studies, and searchable list of patient advocacy groups.
National Organization for Rare Disorders (NORD)
Helps patients find information, access to medication, connections with others affected by rare disease, patient meetings/organizations, and advocacy opportunities.
Global Genes
Offers tools on living with rare disease and/or advocating for rare disease patients.
Undiagnosed Diseases Network
Helps patients and families living with undiagnosed conditions and seeks to provide answers.
American Society of Cell & Gene Therapy (ASCGT)
Provides helpful information to guide you through the challenging journey from diagnosis to pursuing a gene therapy.
“How to Find, Interview and Choose a Patient Advocate: 6 Key Questions to Ask” from verywell
Searchable directories of patient advocates from the National Association of Healthcare Advocacy Consultants and AdvoConnection
FINDING A SUPPORT GROUP
Online support groups for caregivers from caring.com
Online and in-person support group options from the Family Caregiver Alliance
Resources on finding support from The National Alliance on Mental Illness
“Finding Support Programs and Services In Your Area” from The American Cancer Society
