Copycat genes uncover new ways to conserve water

Rice is a staple food for more than half the world’s population. So how do we safeguard it as global climates increase and drought becomes more frequent? This is a question IGI’s Director of Sustainable Agriculture, Brian Staskawicz, is trying to answer. In a new paper from his lab published this week in Plant Physiology, first author and Staskawicz lab member Nicholas Karavolias and the rest of the research team use CRISPR to open a new path towards drought-tolerant rice.
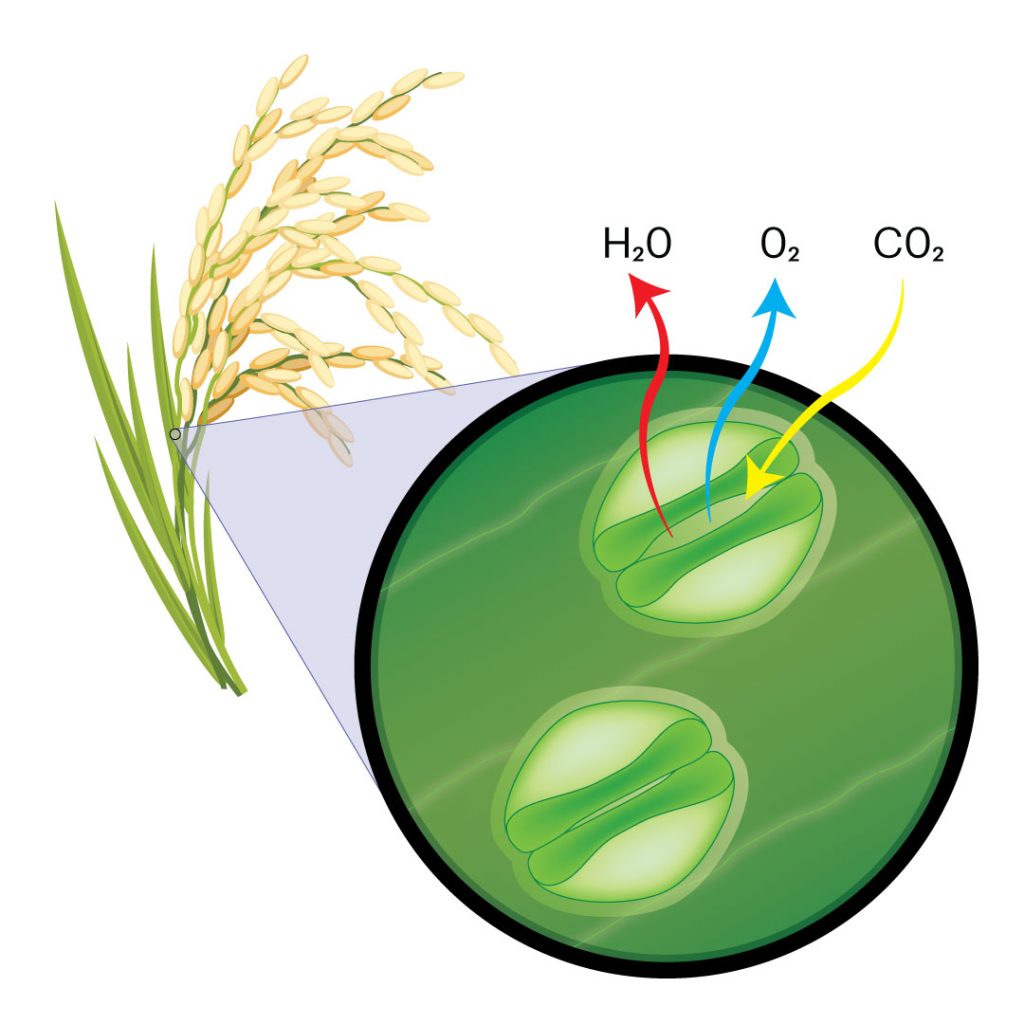
Just like we have pores on our skin, the leaves of rice and other plants have tiny pores called “stomata.” This name comes from the Greek word for “mouth” and is a nod to their appearance and ability to open and close depending on environmental triggers.
Stomata allow the plant to take in carbon dioxide and release the oxygen that we breathe. Plants also use stomata to control their temperature. In a process analogous to human sweating, plants release water through stomata. The process of evaporation helps them lower their temperature.
This leads to the central idea: since rice and other plants lose water through stomata, can we make them more water efficient – and more drought-tolerant – by reducing the total number of stomata?
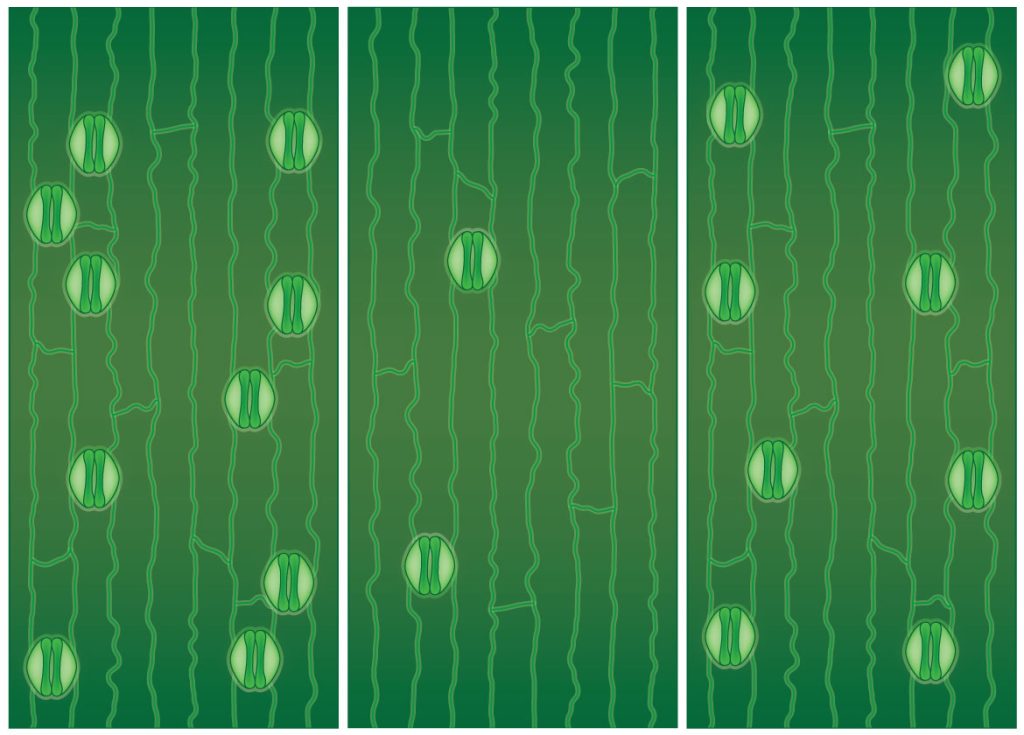
It has been known for some time that the gene STOMAGEN (short for “stomata generator”) is crucial for the development of stomata. Previously, researchers have reduced the number of stomata by disabling or “knocking out” the STOMAGEN gene in rice. This leads to an ~80% reduction in the number of stomata. “The literature was able to show us that reducing the number of stomata could improve water use efficiency,” says Karavolias, “But at a cost.”
Indeed, the loss of so many stomata also dramatically reduces the plant’s ability to do the exchange of carbon dioxide and oxygen that drives photosynthesis, plant growth, and plant yield. It may also reduce plants’ ability to effectively regulate temperature. Could there be another approach to water efficiency?
It is not uncommon for plants to contain more than one copy of the same or very similar genes. In rice, there is a copy, or paralog, of the STOMAGEN gene called EPFL10, which has almost an identical genetic code. Karavolias and the Staskawicz lab team decided to investigate whether EPFL10 might hold the solution.
Using CRISPR, Karavolias and colleagues were able to compare STOMAGEN and EPFL10. Like STOMAGEN, EPFL10 promotes the development of stomata in rice leaves. But its effects are more mild – knocking out EPFL10 reduces the number of stomata, but less dramatically than knocking out STOMAGEN. In effect, gene editing EPFL10 allows for fine tuning the number of stomata.
“We counted the stomata manually,” explains Karavolias.”And the way that we counted it is actually really silly. You paint the undersides of the leaves with clear nail polish, which makes a beautiful impression of the leaf, even the microscopic structures. While you’re waiting for it to dry, you also paint your own nails. That’s essential, it’s in the methods! And then you use double-sided tape to remove the nail polish imprint, which you can then image with a microscope.”

Next, Karavolias and colleagues set out to assess the physiological effects on the rice plants with studies in IGI’s greenhouse. Both knock-outs showed similar increases in water conservation. But what gas exchange, temperature regulation, and yield?
Working in collaboration with Kris Niyogi’s lab at UC Berkeley, the team first looked at gas exchange and photosynthesis.

“We know how important stomata are for maintaining water, transpiring water, and being water efficient, but they’re also important holes for CO2 to come in so plants can do photosynthesis,” says Dhruv Patel-Tupper, a postdoc in the Nyogi lab and co-author on the paper. “I came onto the project to see what was happening with photosynthesis. Is it compromised? How do we coordinate it with other traits, like water use efficiency, that we’re interested in? We used a device that clamps a tiny portion of leaf and makes a perfect temperature- and humidity-controlled chamber to quantify how well they’re doing photosynthesis under different light conditions.”
In Patel-Tupper’s experiments, the STOMAGEN knock-out negatively affected gas exchange, but there was no difference in gas exchange between plants with EPFL10 knocked out and plants with no gene edits. Likewise, the team’s other experiments showed that STOMAGEN knockouts had trouble regulating leaf temperature in some conditions, EPFL10 knockouts were able to regulate their temperature as well as unedited plants in every condition tested. Finally, no differences in yield were observed between the rice lines.
“Rice is one of the most important crops in the world that feed people. It’s also a great model system for lab research as it is easily transformed and gene edited. The results we have so far are very exciting as greenhouse tests have shown a reduction in the density of stomata, but maintain photosynthetic capacity,” says Staskawicz. “However, The real proof for translation is getting it to work in the field. In agriculture, a field trial is the equivalent of a clinical trial.”

The next steps for this project are already underway: testing the effects of the gene edits in rice plants grown in a field trial. Researchers at the International Center for Tropical Agriculture in Colombia have already started growing the edited plants. ICTA is part of the CGIAR network. Down the line, if field trials yield positive results, CGIAR could help distribute drought-tolerant seeds globally. While these experiments were done in rice cultivars typically used for research rather than food, the edits would be straightforward to transfer to rice crop cultivars and other crops with similar gene duplicates.
The Staskawicz lab team is also looking beyond the rice genome, with bioinformatic analysis by research assistant and co-author Kyungyong Seong.
“You see duplications of the same gene in other plants, like lettuces, carrot, and sunflower,” says Karavolias. “So, we can take this line of research forward to other crops as a way to fine-tune stomatal density. It’s also good evidence that these duplicate genes are prime gene-editing targets for other traits as well.”



