Melanie Ott is the Director of the Institute of Virology and Immunology at Gladstone Institutes and a Professor of Medicine at the University of California, San Francisco. Her lab primarily studies the human immunodeficiency virus (HIV-1) and the hepatitis C virus, aiming to understand the molecular interactions between viruses and their target cells to yield targets for new therapeutic drugs. In December, she talked with IGI science writer Hope Henderson about her current research on COVID-19 therapeutic targets.
Tell me about your COVID-19 Project.
This is a very close collaboration with Nevan Krogan. The basic idea is that we’re going after host proteins that are important for the life cycle of SARS-CoV-2, the virus that causes COVID-19. Viruses cannot survive without support from the cell they infect — the host cell. They’re critically dependent on the host cell and its proteins. If we can figure out which proteins are the most crucial lifelines for the virus, we might be able to create an antiviral treatment by cutting these lifelines. This is different from usual antivirals because those target the virus itself.

How do you find host factors?
Nevan has identified more than 300 of these factors by mass spectrometry. The fact that he published basically a source code within a few months of the pandemic shows that this is something that he has perfected. What we propose in this grant is systematically testing each of the 300 plus factors that have been identified as interacting with SARS-CoV-2 proteins to determine which are crucial for the viral life cycle.
How do you figure out which host proteins are crucial?
Our emphasis is to do this in the way that is closest to what it would be like in an actual person, so infect lung cells and lung organoids in the lab. The second part is to figure out the best ways to use CRISPR to turn genes off in these cells and organoids. By turning off host genes one by one, we can figure out which ones are most important for the virus.
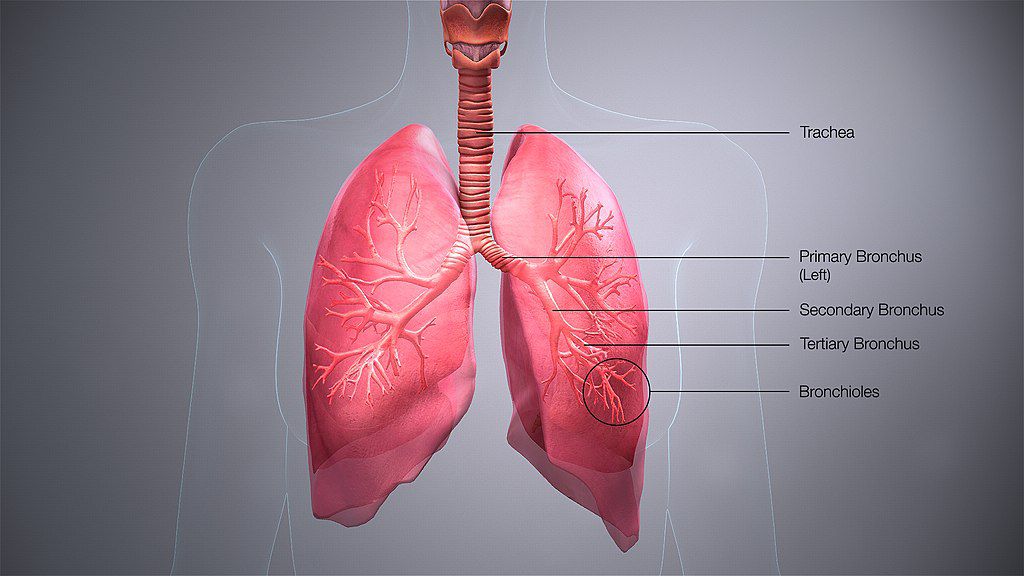
What exactly is an organoid?
An organoid is a mini organ in a petri dish. It’s not quite an organ – it doesn’t have, for example, blood vessels, but it is three-dimensional and usually presents all relevant epithelial cell types. My lab has developed organoid models for different tissues over the last few years. We make human airway organoids by receiving from our clinical partners pieces of lungs usually from resections. We isolate the stem cells and grow them in a special environment so they produce some of the different types of cells you see in a real patient’s airway grown in a three-dimensional structure.
What are the advantages of using organoids?
Normally, scientists use cancer cell lines to study infectious diseases because they grow well in the lab, but they are different from the cells the virus infects in people. There’s a lot we can learn from studying cancer cell lines, but there is also a big problem that we have in science where things look very promising in the lab and even in mice, but then you try them in patients and they fail. Cancer cells are really different from normal cells in some important ways that can contribute to that. So, researchers are very excited about organoids because they have different, mature, non-cancerous cell types and the three-dimensional structure. You can also grow organoids from different people, so you can test things with some of the genetic variability that you see in a population.
What do you do when you think you’ve found an important host protein?
After testing proteins in organoids, we test promising hits in bronchial epithelial cells that are isolated from people directly. These are a mix of the kinds of cells that line the bronchi, or, airways.
This is more of a one-shot experiment that’s even closer to what is going on in people, because you’re not growing and multiplying the cells and helping them mature – you’re using cells that are already mature, direct from people. Which also means you have a limited number! That’s why we save them for the most promising hits.
The bronchial epithelial cells grow in a two-dimensional layer. In a person, the bronchial epithelial cells line the airway, so they are exposed to air on one side and our body fluids on the other. Most cells in labs are grown totally submerged in liquid. We grow these cells in a special set-up called an air-liquid interface model, where the upper part of the cell is exposed to the air and the bottom part is in liquid.
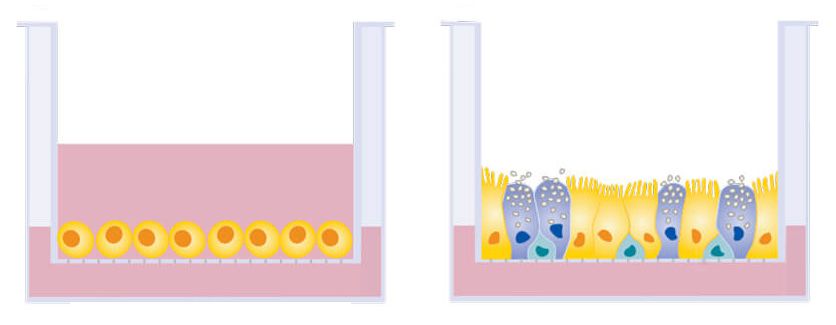
How do the cells respond?
The cells get really happy. They are in an environment that’s a lot like an airway now, and they actually promote COVID-19 infection more strongly when grown this way than grown in more traditional ways. So where in the project are you now? The initial hope when we wrote the project was, we will be done in two months. But no surprise, we’re still very much working on it! We got into SARS-CoV-2 research because we were working on lung organoids already. We had them in our incubator and they got beautifully infected with influenza! But it turned out that it is much harder to effectively infect them with SARS-CoV-2, and that’s part of why the project is taking longer than we planned.
Why is it harder to infect the airway?
It probably indicates that we’ve evolved defense mechanisms in the airway to not fall completely prey to coronaviruses. Once the virus gains access, it can distribute and disseminate and go into other organs pretty easily, and then we have big problems. The airway epithelium is the first layer of defense. So, in my mind it speaks to the fact that coronaviruses have been around for a while.
What will you learn from manipulating genes with CRISPR?
We can figure out which host proteins the virus really needs, because when we get rid of them with CRISPR, the virus can’t replicate. We can also find what we call restriction factors – host proteins that stop the virus from replicating. When we get rid of them, the virus replicates much more. Finding proteins in both of these groups can help us develop new treatments for COVID-19.
What would be your ideal outcome from the project?
The ideal outcome would be to find a drug target that is effective against SARS-CoV-2 but also other coronaviruses. The advantage of targeting host factors is that some are likely shared between multiple viruses. Finding those host factors can help in developing pan-antivirals which hopefully will be ready to tackle the next pandemic virus on the horizon.
How did you first come to work with Nevan Krogan?
I don’t know anymore – we’ve been collaborating for so long! He is just a wonderful colleague, and we have this shared interest in host-virus interactions.
One more question – have you developed any new quarantine hobbies?
I became a Peloton fan! My husband has a Peloton bike and he was always a very avid fan, but I was always a bit dismissive of it. I used to be very active with Bikram yoga but since March, the Peloton has saved my life. I’m also cooking a lot and maybe eating too much!
This work is funded by the Laboratory for Genomics Research (LGR), a collaboration between UC Berkeley/UCSF (IGI) and GlaxoSmithKline.